Structural insights into double-stranded RNA recognition and transport by SID-1
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
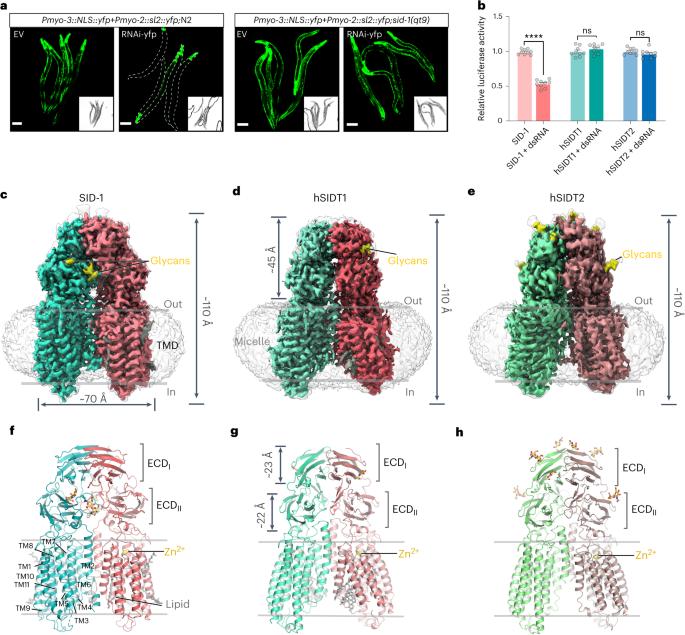
RNA uptake by cells is critical for RNA-mediated gene interference (RNAi) and RNA-based therapeutics. In Caenorhabditis elegans, RNAi is systemic as a result of SID-1-mediated double-stranded RNA (dsRNA) across cells. Despite the functional importance, the underlying mechanisms of dsRNA internalization by SID-1 remain elusive. Here we describe cryogenic electron microscopy structures of SID-1, SID-1–dsRNA complex and human SID-1 homologs SIDT1 and SIDT2, elucidating the structural basis of dsRNA recognition and import by SID-1. The homodimeric SID-1 homologs share conserved architecture, but only SID-1 possesses the molecular determinants within its extracellular domains for distinguishing dsRNA from single-stranded RNA and DNA. We show that the removal of the long intracellular loop between transmembrane helix 1 and 2 attenuates dsRNA uptake and systemic RNAi in vivo, suggesting a possible endocytic mechanism of SID-1-mediated dsRNA internalization. Our study provides mechanistic insights into dsRNA internalization by SID-1, which may facilitate the development of dsRNA applications based on SID-1. In C. elegans, systemic RNAi is initiated by SID-1-mediated dsRNA internalization. Here the authors present cryo-EM structures of SID-1 homologs and the SID-1–dsRNA complex, elucidating the structural basis for dsRNA recognition and uptake by SID-1.
SID-1识别和运输双链RNA的结构启示
细胞摄取 RNA 对 RNA 介导的基因干扰(RNAi)和基于 RNA 的疗法至关重要。在秀丽隐杆线虫中,RNAi 是 SID-1 介导的双链 RNA(dsRNA)跨细胞的系统性结果。尽管dsRNA在功能上非常重要,但SID-1内化dsRNA的基本机制仍然难以捉摸。在这里,我们描述了 SID-1、SID-1-dsRNA 复合物以及人类 SID-1 同源物 SIDT1 和 SIDT2 的低温电子显微镜结构,阐明了 SID-1 识别和导入 dsRNA 的结构基础。同源二聚体 SID-1 具有保守的结构,但只有 SID-1 的胞外结构域具有分子决定因素,可将 dsRNA 与单链 RNA 和 DNA 区分开来。我们的研究表明,去除跨膜螺旋 1 和 2 之间的长胞内环可减少体内 dsRNA 的摄取和系统性 RNAi,这表明 SID-1 介导的 dsRNA 内化可能存在内细胞机制。我们的研究提供了 SID-1 内化 dsRNA 的机理见解,这可能有助于开发基于 SID-1 的 dsRNA 应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


