Alternative splicing of CARM1 regulated by LincGET-guided paraspeckles biases the first cell fate in mammalian early embryos
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
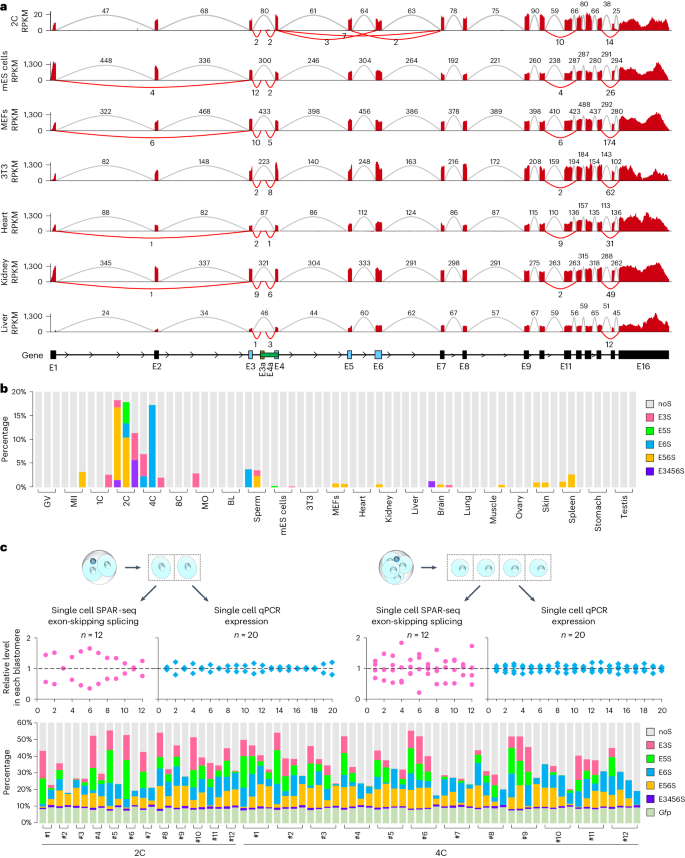
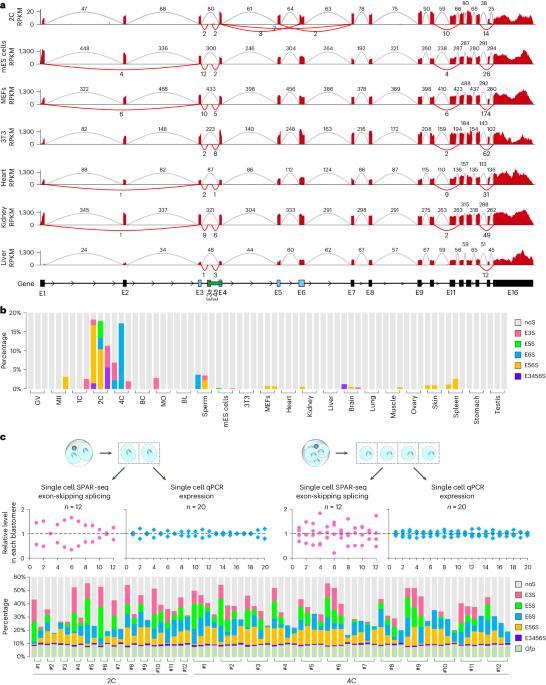
The heterogeneity of CARM1 controls first cell fate bias during early mouse development. However, how this heterogeneity is established is unknown. Here, we show that Carm1 mRNA is of a variety of specific exon-skipping splicing (ESS) isoforms in mouse two-cell to four-cell embryos that contribute to CARM1 heterogeneity. Disruption of paraspeckles promotes the ESS of Carm1 precursor mRNAs (pre-mRNAs). LincGET, but not Neat1, is required for paraspeckle assembly and inhibits the ESS of Carm1 pre-mRNAs in mouse two-cell to four-cell embryos. We further find that LincGET recruits paraspeckles to the Carm1 gene locus through HNRNPU. Interestingly, PCBP1 binds the Carm1 pre-mRNAs and promotes its ESS in the absence of LincGET. Finally, we find that the ESS seen in mouse two-cell to four-cell embryos decreases CARM1 protein levels and leads to trophectoderm fate bias. Our findings demonstrate that alternative splicing of CARM1 has an important role in first cell fate determination. The heterogeneity of CARM1 for the first cell fate bias in mice arises from exon-skipping splicing of Carm1 pre-mRNAs, which is regulated by the competition between LincGET-paraspeckles and splicing speckles for binding to the Carm1 locus.
受 LincGET 引导的副斑块调控的 CARM1 的替代剪接会影响哺乳动物早期胚胎中第一个细胞的命运
在小鼠早期发育过程中,CARM1 的异质性控制着第一细胞的命运偏向。然而,这种异质性是如何形成的尚不清楚。在这里,我们发现在小鼠两细胞到四细胞胚胎中,Carm1 mRNA具有多种特异的外显子剪接(ESS)异构体,这些异构体导致了CARM1的异质性。旁斑的破坏会促进 Carm1 前体 mRNA(pre-mRNA)的ESS。在小鼠两细胞到四细胞胚胎中,副颈组装需要 LincGET,但不需要 Neat1,LincGET 可抑制 Carm1 前体 mRNA 的ESS。我们进一步发现,LincGET 通过 HNRNPU 将副壁细胞招募到 Carm1 基因位点。有趣的是,在没有 LincGET 的情况下,PCBP1 也能结合 Carm1 前 mRNA 并促进其ESS。最后,我们发现在小鼠两细胞到四细胞胚胎中出现的ESS会降低CARM1蛋白水平,并导致滋养层命运偏向。我们的研究结果表明,CARM1 的替代剪接在第一细胞命运决定中起着重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


