Concerted SUMO-targeted ubiquitin ligase activities of TOPORS and RNF4 are essential for stress management and cell proliferation
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
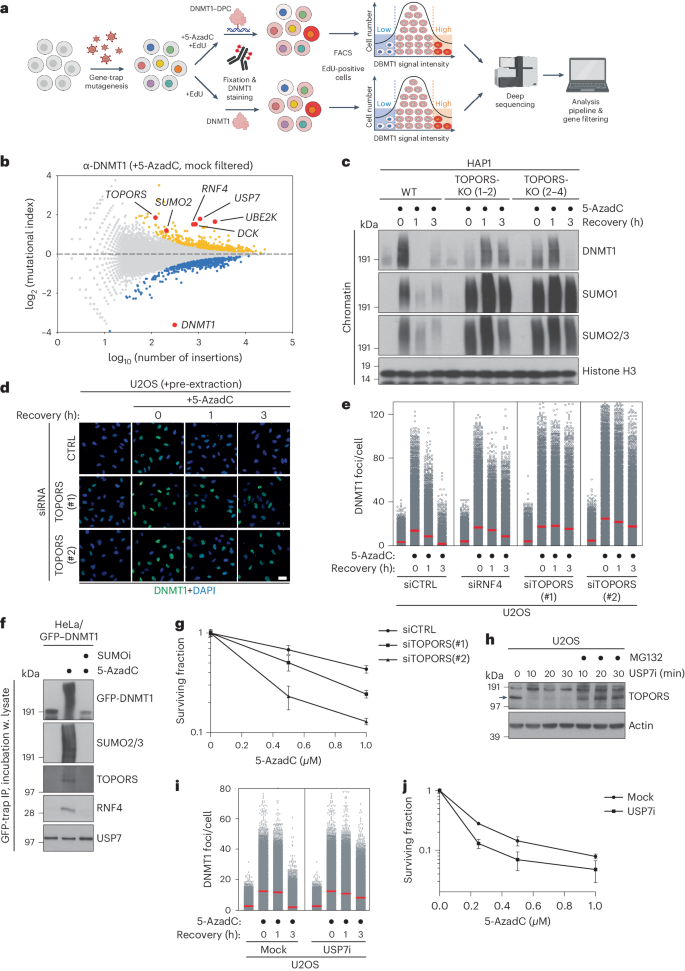
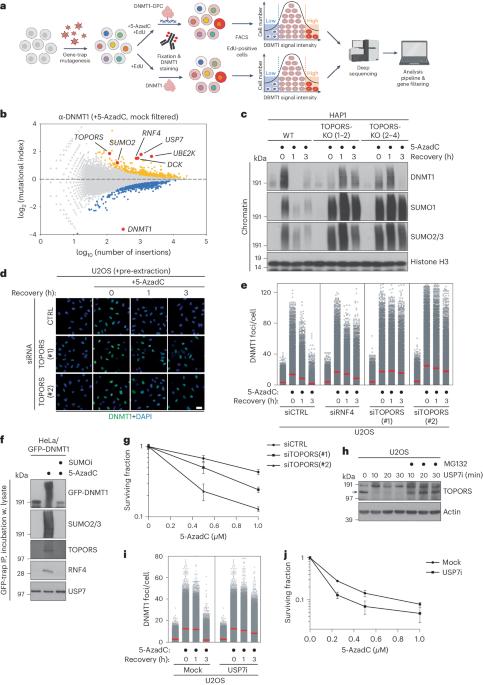
Protein SUMOylation provides a principal driving force for cellular stress responses, including DNA–protein crosslink (DPC) repair and arsenic-induced PML body degradation. In this study, using genome-scale screens, we identified the human E3 ligase TOPORS as a key effector of SUMO-dependent DPC resolution. We demonstrate that TOPORS promotes DPC repair by functioning as a SUMO-targeted ubiquitin ligase (STUbL), combining ubiquitin ligase activity through its RING domain with poly-SUMO binding via SUMO-interacting motifs, analogous to the STUbL RNF4. Mechanistically, TOPORS is a SUMO1-selective STUbL that complements RNF4 in generating complex ubiquitin landscapes on SUMOylated targets, including DPCs and PML, stimulating efficient p97/VCP unfoldase recruitment and proteasomal degradation. Combined loss of TOPORS and RNF4 is synthetic lethal even in unstressed cells, involving defective clearance of SUMOylated proteins from chromatin accompanied by cell cycle arrest and apoptosis. Our findings establish TOPORS as a STUbL whose parallel action with RNF4 defines a general mechanistic principle in crucial cellular processes governed by direct SUMO–ubiquitin crosstalk. Liu et al. reveal that human TOPORS is a SUMO1-selective SUMO-targeted ubiquitin ligase (STUbL). The parallel action of TOPORS and the STUbL RNF4 defines a general mechanistic principle governing pathways driven by direct SUMO–ubiquitin crosstalk.
TOPORS和RNF4的协同SUMO靶向泛素连接酶活性对应激管理和细胞增殖至关重要
蛋白质 SUMOylation 是细胞应激反应的主要驱动力,包括 DNA 蛋白交联(DPC)修复和砷诱导的 PML 体降解。在这项研究中,我们利用基因组规模的筛选,发现人类 E3 连接酶 TOPORS 是 SUMO 依赖性 DPC 修复的关键效应物。我们证明,TOPORS 作为一种 SUMO 靶向泛素连接酶(STUbL),通过其 RING 结构域将泛素连接酶活性与通过 SUMO 相互作用基序(类似于 STUbL RNF4)将多 SUMO 结合起来,从而促进了 DPC 的修复。从机理上讲,TOPORS是一种SUMO1选择性STUbL,它与RNF4互补,在SUMO化靶标(包括DPCs和PML)上生成复杂的泛素景观,刺激p97/VCP折叠酶的有效招募和蛋白酶体降解。TOPORS 和 RNF4 的联合缺失即使在未受激细胞中也是合成致死性的,涉及染色质中 SUMOylated 蛋白的缺陷清除,并伴有细胞周期停滞和细胞凋亡。我们的研究结果确立了 TOPORS 作为 STUbL 的地位,它与 RNF4 的平行作用定义了由 SUMO-ubiquitin 直接串联调节的关键细胞过程中的一般机制原理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


