H2O2-mediated electrosynthesis of nitrate from air
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Renewable electricity-driven electrochemical nitrogen oxidation is a promising alternative to traditional Haber–Bosch and Ostwald processes to directly synthesize nitrate from nitrogen. However, its efficiency is hindered by strong competition from the oxygen evolution reaction in aqueous environments, along with a deficiency in standardized testing protocols. Here we present an efficient approach for nitrogen oxidation, substituting the oxygen evolution reaction with hydroxyl radicals (·OH) generated through hydrogen peroxide decomposition to serve as an active oxygen source. Electrochemical tests demonstrate that the nitrogen oxidation, facilitated by ·OH, can achieve a Faradaic efficiency of 25.6% and a nitrate yield of 8.3 nmol s−1 cm−2. Furthermore, we employed in situ electrochemical mass spectrometry, gas-phase infrared and electron paramagnetic resonance spectroscopy to establish a comprehensive set of benchmarks to confirm the authenticity of nitrogen activation and to examine the reaction mechanism mediated by ·OH. Techno-economic analysis underscores the promising feasibility and sustainable economic value of the presented method. Renewable electricity-driven nitrogen oxidation is a green alternative to Haber–Bosch and Ostwald processes, but it is challenging to effectively steer oxygen intermediates towards the nitrogen oxidation reaction pathway. Now, to mitigate competing oxygen evolution and improve nitrogen oxidation efficiency, the use of hydroxyl radicals as the nitrogen oxidant is proposed.
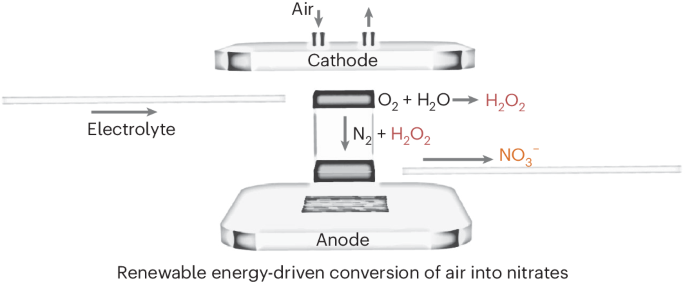
以 H2O2- 为媒介从空气中电合成硝酸盐
可再生电力驱动的电化学氮氧化法是传统的哈伯-博什法和奥斯特瓦尔德法的一种很有前途的替代方法,可直接从氮气中合成硝酸盐。然而,由于水环境中氧进化反应的强烈竞争以及标准化测试协议的缺乏,其效率受到了阻碍。在这里,我们提出了一种高效的氮氧化方法,用过氧化氢分解产生的羟基自由基(-OH)取代氧进化反应作为活性氧源。电化学测试表明,在 -OH 的促进下,氮氧化物的法拉第效率可达 25.6%,硝酸盐产量为 8.3 nmol s-1 cm-2。此外,我们还利用原位电化学质谱法、气相红外光谱法和电子顺磁共振光谱法建立了一套全面的基准,以确认氮活化的真实性,并研究由 -OH 介导的反应机制。技术经济分析强调了该方法的可行性和可持续经济价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


