A pollen selection system links self and interspecific incompatibility in the Brassicaceae
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
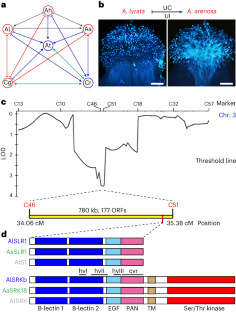
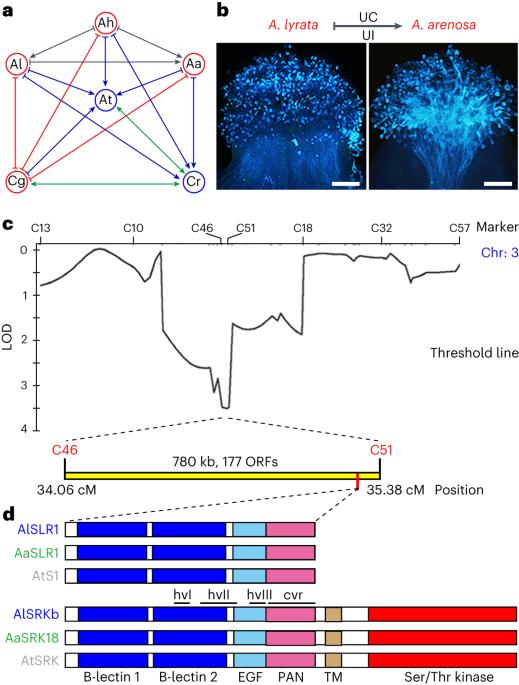
Self-incompatibility and recurrent transitions to self-compatibility have shaped the extant mating systems underlying the nonrandom mating critical for speciation in angiosperms. Linkage between self-incompatibility and speciation is illustrated by the shared pollen rejection pathway between self-incompatibility and interspecific unilateral incompatibility (UI) in the Brassicaceae. However, the pollen discrimination system that activates this shared pathway for heterospecific pollen rejection remains unknown. Here we show that Stigma UI3.1, the genetically identified stigma determinant of UI in Arabidopsis lyrata × Arabidopsis arenosa crosses, encodes the S-locus-related glycoprotein 1 (SLR1). Heterologous expression of A. lyrata or Capsella grandiflora SLR1 confers on some Arabidopsis thaliana accessions the ability to discriminate against heterospecific pollen. Acquisition of this ability also requires a functional S-locus receptor kinase (SRK), whose ligand-induced dimerization activates the self-pollen rejection pathway in the stigma. SLR1 interacts with SRK and interferes with SRK homomer formation. We propose a pollen discrimination system based on competition between basal or ligand-induced SLR1–SRK and SRK–SRK complex formation. The resulting SRK homomer levels would be sensed by the common pollen rejection pathway, allowing discrimination among conspecific self- and cross-pollen as well as heterospecific pollen. Our results establish a mechanistic link at the pollen recognition phase between self-incompatibility and interspecific incompatibility. Through genetic and molecular analyses of interspecific stigma–pollen interactions, the authors show that Brassicaceae plants use an integrated pollen discrimination system and a shared pollen rejection pathway to reject conspecific self-pollen and heterospecific pollen. This establishes a mechanistic link between self-incompatibility and speciation in this clade.
将十字花科植物自交不亲和与种间不亲和联系起来的花粉选择系统
自相容性和向自相容性的反复转变形成了现存的交配系统,而非随机交配是被子植物物种分化的关键。十字花科植物中自交不亲和与种间单侧不亲和(UI)之间共享的花粉排斥途径说明了自交不亲和与物种分化之间的联系。然而,激活这一共享的异种花粉排斥途径的花粉鉴别系统仍然未知。在这里,我们发现拟南芥(Arabidopsis lyrata)×拟南芥(Arabidopsis arenosa)杂交的柱头UI3.1编码S-焦点相关糖蛋白1(SLR1)。异源表达拟南芥或大花拟南芥 SLR1 可使某些拟南芥品种具有辨别异质花粉的能力。这种能力的获得还需要一个功能性的 S-焦点受体激酶(SRK),其配体诱导的二聚体激活了柱头中的自花粉排斥途径。SLR1 与 SRK 相互作用,并干扰 SRK 同源体的形成。我们提出了一种基于基础或配体诱导的 SLR1-SRK 与 SRK-SRK 复合物形成之间竞争的花粉识别系统。由此产生的 SRK 同源物水平会被共同的花粉排斥途径感知,从而在同种自花粉、异花粉和异种花粉之间进行区分。我们的研究结果在花粉识别阶段建立了自交不亲和与种间不亲和之间的机理联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


