Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The increasing prevalence of antimicrobial resistance in Staphylococcus aureus necessitates alternative therapeutic approaches. Neutrophils play a crucial role in the fight against S. aureus but suffer from deficiencies in function leading to increased infection. Here we report a nanoparticle-mediated immunotherapy aimed at potentiating neutrophils to eliminate S. aureus. The nanoparticles consist of naftifine, haemoglobin (Hb) and a red blood cell membrane coating. Naftifine disrupts staphyloxanthin biosynthesis, Hb reduces bacterial hydrogen sulfide levels and the red blood cell membrane modifies bacterial lipid composition. Collectively, the nanoparticles can sensitize S. aureus to host oxidant killing. Furthermore, in the infectious microenvironment, Hb triggers lipid peroxidation in S. aureus, promoting neutrophil chemotaxis. Oxygen supplied by Hb can also significantly enhance the bactericidal capability of the recruited neutrophils by restoring neutrophil respiratory burst via hypoxia relief. This multimodal nanoimmunotherapy demonstrates excellent therapeutic efficacy in treating antimicrobial-resistant S. aureus persisters, biofilms and S. aureus-induced infection in mice. Antimicrobial resistance is becoming more prevalent. Here the authors use multimodal nanoparticles to modulate the infected microenvironment, recruit neutrophils and alleviate hypoxia to restore neutrophil function, demonstrating therapeutic efficacy against MRSA infections in mice.
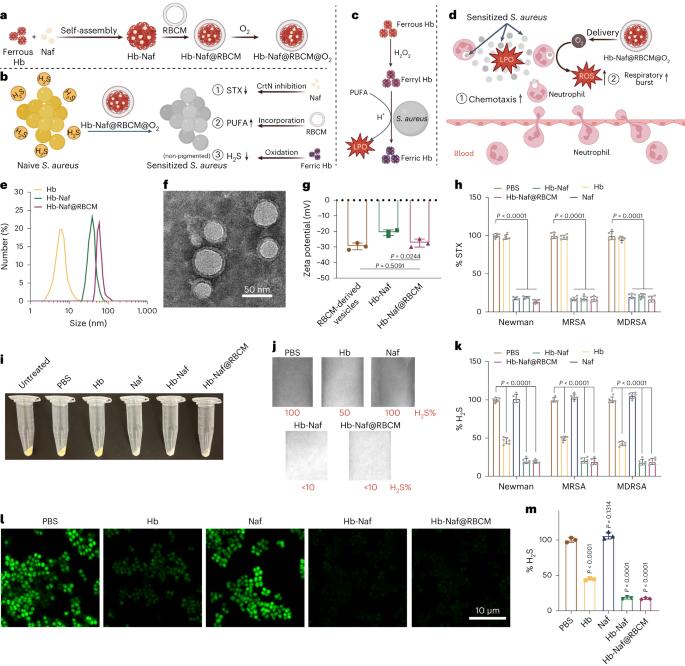
多模式纳米免疫疗法吸引中性粒细胞消除金黄色葡萄球菌感染
金黄色葡萄球菌的抗菌药耐药性日益普遍,因此需要采用其他治疗方法。中性粒细胞在抗击金黄色葡萄球菌的过程中发挥着至关重要的作用,但其功能缺陷会导致感染增加。在此,我们报告了一种纳米颗粒介导的免疫疗法,旨在增强中性粒细胞消除金黄色葡萄球菌的能力。纳米颗粒由萘替芬、血红蛋白(Hb)和红细胞膜涂层组成。萘替芬可破坏钉螺黄素的生物合成,血红蛋白可降低细菌的硫化氢水平,红细胞膜可改变细菌的脂质组成。总之,纳米粒子可使金黄色葡萄球菌对宿主氧化剂的杀伤作用敏感。此外,在感染性微环境中,血红蛋白会引发金黄色葡萄球菌的脂质过氧化反应,促进中性粒细胞趋化。Hb 提供的氧气还能通过缓解缺氧状态恢复中性粒细胞的呼吸爆发,从而显著增强招募的中性粒细胞的杀菌能力。这种多模式纳米免疫疗法在治疗耐抗菌金黄色葡萄球菌宿主、生物膜和金黄色葡萄球菌诱导的小鼠感染方面显示出卓越的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


