Phosphorylation of Mad1 at serine 18 by Mps1 is required for the full virulence of rice blast fungus, Magnaporthe oryzae
IF 4.8
1区 农林科学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
The spindle assembly checkpoint (SAC) proteins are conserved among eukaryotes safeguarding chromosome segregation fidelity during mitosis. However, their biological functions in plant‐pathogenic fungi remain largely unknown. In this study, we found that the SAC protein MoMad1 in rice blast fungus (
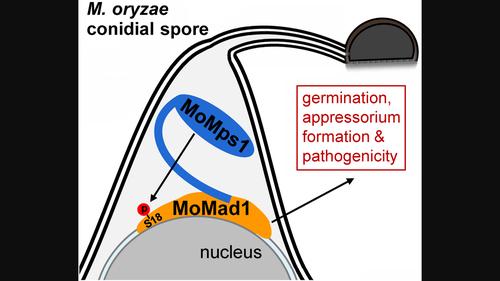
稻瘟病真菌 Magnaporthe oryzae 的完全毒力需要 Mps1 在丝氨酸 18 处磷酸化 Mad1
纺锤体装配检查点(SAC)蛋白是真核生物中的保守蛋白,在有丝分裂过程中保障染色体分离的保真度。然而,它们在植物病原真菌中的生物学功能在很大程度上仍然未知。本研究发现,稻瘟病真菌(Magnaporthe oryzae)中的 SAC 蛋白 MoMad1 定位于核膜上,对于稻瘟病真菌的无性生长和耐受微管解聚剂处理是不可或缺的。MoMad1 在与 M. oryzae 感染相关的发育和致病性中发挥着重要作用。M. oryzae 中的单极纺锤体 1 同源物(MoMps1)通过其 N 端结构域与 MoMad1 相互作用,并在 Ser-18 处磷酸化 MoMad1。这种磷酸化是维持 MoMad1 蛋白丰度和 M. oryzae 完全毒力所必需的。与缺失 MoMad1 相似,用 Mps1-IN-1(一种 Mps1 抑制剂)处理也会导致附着体形成受阻和 M. oryzae 毒力下降,而这些缺陷都依赖于它对 MoMad1 Ser-18 磷酸化的抑制作用。因此,我们的研究表明了Mad1在稻瘟病真菌致病性中的功能,并揭示了通过Mps1阻断Mad1磷酸化来控制作物真菌病害的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular plant pathology
生物-植物科学
CiteScore
9.40
自引率
4.10%
发文量
120
审稿时长
6-12 weeks
期刊介绍:
Molecular Plant Pathology is now an open access journal. Authors pay an article processing charge to publish in the journal and all articles will be freely available to anyone. BSPP members will be granted a 20% discount on article charges. The Editorial focus and policy of the journal has not be changed and the editorial team will continue to apply the same rigorous standards of peer review and acceptance criteria.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


