Brillouin microscopy monitors rapid responses in subcellular compartments
IF 15.7
Q1 OPTICS
引用次数: 0
Abstract
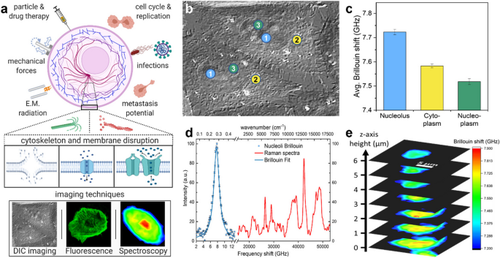
Measurements and imaging of the mechanical response of biological cells are critical for understanding the mechanisms of many diseases, and for fundamental studies of energy, signal and force transduction. The recent emergence of Brillouin microscopy as a powerful non-contact, label-free way to non-invasively and non-destructively assess local viscoelastic properties provides an opportunity to expand the scope of biomechanical research to the sub-cellular level. Brillouin spectroscopy has recently been validated through static measurements of cell viscoelastic properties, however, fast (sub-second) measurements of sub-cellular cytomechanical changes have yet to be reported. In this report, we utilize a custom multimodal spectroscopy system to monitor for the very first time the rapid viscoelastic response of cells and subcellular structures to a short-duration electrical impulse. The cytomechanical response of three subcellular structures - cytoplasm, nucleoplasm, and nucleoli - were monitored, showing distinct mechanical changes despite an identical stimulus. Through this pioneering transformative study, we demonstrate the capability of Brillouin spectroscopy to measure rapid, real-time biomechanical changes within distinct subcellular compartments. Our results support the promising future of Brillouin spectroscopy within the broad scope of cellular biomechanics.
布里渊显微镜监测亚细胞区的快速反应
生物细胞机械响应的测量和成像对于了解许多疾病的机理以及能量、信号和力传导的基础研究至关重要。最近出现的布里渊显微镜是一种功能强大的非接触、无标记方法,可对局部粘弹性进行非侵入、非破坏性评估,为将生物力学研究范围扩展到亚细胞水平提供了机会。最近,布里渊光谱法通过静态测量细胞粘弹性特性得到了验证,然而,亚细胞细胞力学变化的快速(亚秒级)测量尚未见报道。在本报告中,我们利用定制的多模态光谱系统首次监测了细胞和亚细胞结构对短时电脉冲的快速粘弹性响应。我们监测了三种亚细胞结构--细胞质、核质和核小体--的细胞机械响应,结果显示,尽管受到相同的刺激,它们还是发生了明显的机械变化。通过这项开创性的变革研究,我们展示了布里渊光谱法测量不同亚细胞区室内快速、实时生物力学变化的能力。我们的研究结果支持了布里渊光谱学在细胞生物力学领域的广阔前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


