Chinese medicinal formula Fu Xin decoction against chronic heart failure by inhibiting the NLRP3/caspase-1/GSDMD pyroptotic pathway
IF 7.5
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
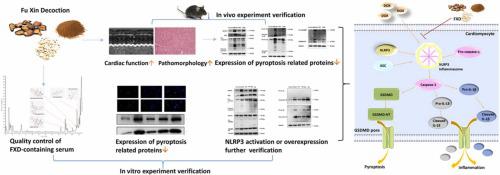
Various heart diseases ultimately lead to chronic heart failure (CHF). In CHF, the inflammatory response is associated with pyroptosis, which is mediated by the NOD-like receptor protein 3 (NLRP3) inflammasome. Fu Xin decoction (FXD) is commonly used in clinical practice to treat CHF and improve inflammatory conditions. However, the specific pharmacological mechanisms of action for FXD in these processes have yet to be fully understood. The objective of this study was to examine the protective mechanism of FXT against CHF, both in H9c2 cells and mice. A CHF mouse model was established, and the effect of FXD was observed via gavage. Cardiac function was evaluated using echocardiography, while serum BNP and LDH levels were analyzed to assess the severity of CHF. Hematoxylin and eosin staining (H&E) and Masson staining were performed to evaluate myocardial pathological changes, and TdT-mediated dUTP Nick-End Labeling staining was used to detect DNA damage. Additionally, doxorubicin was utilized to induce myocardial cell injury in H9c2 cells, establishing a relevant model. CCK8 was used to observe cell viability and detect LDH levels in the cell supernatant. Subsequently, the expression of pyroptosis-related proteins was detected using immunohistochemistry, immunofluorescence, and western blotting. Finally, the pharmacological mechanism of FXD against CHF was further validated by treating H9c2 cells with an NLRP3 activator and inducing NLRP3 overexpression. According to current research findings, echocardiography demonstrated a significant improvement of cardiac function by FXD, accompanied by reduced levels of BNP and LDH, indicating the amelioration of cardiac injury in CHF mice. FXD exhibited the ability to diminish serum CRP and MCP inflammatory markers in CHF mice. The results of HE and Masson staining analyses revealed a significant reduction in pathological damage of the heart tissue following FXD treatment. The CCK8 assay demonstrated the ability of FXD to enhance H9c2 cell viability, improve cell morphology, decrease LDH levels in the cell supernatant, and alleviate cell damage. Immunohistochemistry, Western blotting, and immunofluorescence staining substantiated the inhibitory effect of FXD on the NLRP3/caspase-1/GSDMD pyroptosis signaling pathway in both CHF and H9c2 cell injury models. Ultimately, the administration of the NLRP3 activator (Nigericin) and the overexpression of NLRP3 counteract the effects of FXD on cardiac protection and pyroptosis inhibition in vitro. FXD exhibits a cardioprotective effect, improving CHF and alleviating pyroptosis by inhibiting the NLRP3/caspase-1/GSDMD pathway.
通过抑制NLRP3/caspase-1/GSDMD热解途径防治慢性心力衰竭的中药方剂阜新煎剂
各种心脏疾病最终都会导致慢性心力衰竭(CHF)。在慢性心力衰竭中,炎症反应与由 NOD 样受体蛋白 3(NLRP3)炎性基因组介导的热蛋白沉积有关。阜新煎膏剂(FXD)在临床上常用于治疗 CHF 和改善炎症状况。然而,阜新煎膏剂在这些过程中的具体药理作用机制尚未完全明了。本研究旨在研究 FXT 对 H9c2 细胞和小鼠 CHF 的保护机制。研究建立了 CHF 小鼠模型,并通过灌胃观察 FXD 的作用。通过超声心动图评估心功能,同时分析血清 BNP 和 LDH 水平以评估 CHF 的严重程度。采用苏木精和伊红染色法(H&E)和马森染色法评估心肌病理变化,并用TdT介导的dUTP镍末端标记染色法检测DNA损伤。此外,还利用多柔比星诱导 H9c2 细胞心肌细胞损伤,建立了相关模型。用 CCK8 观察细胞活力并检测细胞上清液中的 LDH 水平。随后,使用免疫组化、免疫荧光和 Western 印迹法检测了热蛋白相关蛋白的表达。最后,通过用NLRP3激活剂处理H9c2细胞并诱导NLRP3过表达,进一步验证了FXD抗CHF的药理机制。根据目前的研究结果,超声心动图显示 FXD 显著改善了 CHF 小鼠的心功能,同时降低了 BNP 和 LDH 水平,这表明 FXD 改善了 CHF 小鼠的心脏损伤。FXD 还能降低 CHF 小鼠血清 CRP 和 MCP 炎症指标。HE 和 Masson 染色分析结果显示,FXD 治疗后心脏组织的病理损伤明显减轻。CCK8 试验表明,FXD 能够提高 H9c2 细胞的存活率,改善细胞形态,降低细胞上清液中的 LDH 水平,减轻细胞损伤。免疫组化、Western 印迹和免疫荧光染色证实了 FXD 在 CHF 和 H9c2 细胞损伤模型中对 NLRP3/caspase-1/GSDMD脓毒症信号通路的抑制作用。最终,给予 NLRP3 激活剂(尼日利辛)和过表达 NLRP3 可抵消 FXD 在体外对心脏保护和热蛋白沉积抑制的作用。FXD 具有心脏保护作用,通过抑制 NLRP3/caspase-1/GSDMD通路改善 CHF 并减轻热蛋白沉积。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.90
自引率
2.70%
发文量
1621
审稿时长
48 days
期刊介绍:
Biomedicine & Pharmacotherapy stands as a multidisciplinary journal, presenting a spectrum of original research reports, reviews, and communications in the realms of clinical and basic medicine, as well as pharmacology. The journal spans various fields, including Cancer, Nutriceutics, Neurodegenerative, Cardiac, and Infectious Diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


