Plasma‐catalytic CO2 methanation over NiO/bentonite catalysts prepared by solution combustion synthesis
IF 2.9
3区 物理与天体物理
Q2 PHYSICS, APPLIED
引用次数: 0
Abstract
A solution combustion synthesis (SCS) process to prepare nickel catalyst over bentonite (NiO/Ben) is reported. Compared to the traditional impregnation method, NiO/ben produced by SCS has smaller nickel particle size and higher dispersion. With a metal loading of 20 wt%, the afforded NiO/Ben demonstrates excellent catalytic activity for CO
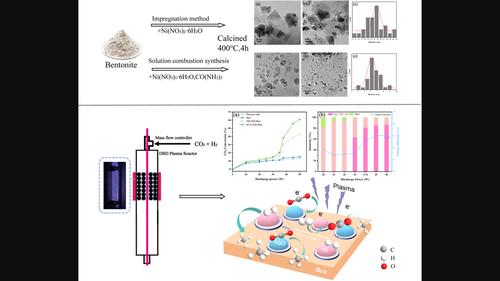
溶液燃烧合成法制备的 NiO/膨润土催化剂上的等离子催化二氧化碳甲烷化
报告采用溶液燃烧合成(SCS)工艺制备膨润土镍催化剂(NiO/Ben)。与传统的浸渍法相比,溶液燃烧合成法制备的镍氧化物/苄具有更小的镍粒径和更高的分散性。在金属含量为 20 wt% 的情况下,NiO/Ben 在介质阻挡放电反应器中的二氧化碳甲烷化过程中表现出优异的催化活性。在特定的放电条件下(原料气中 H2:CO2 的比例为 5,放电输入功率为 45 W,气体时空速度为 11 320 h-1),CO2 转化率和 CH4 选择性分别高达 55.8% 和 84.6%。在等离子体配置条件下,镍元素与支撑物之间的强相互作用在 CO2 甲烷化过程中发挥了重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Plasma Processes and Polymers
物理-高分子科学
CiteScore
6.60
自引率
11.40%
发文量
150
审稿时长
3 months
期刊介绍:
Plasma Processes & Polymers focuses on the interdisciplinary field of low temperature plasma science, covering both experimental and theoretical aspects of fundamental and applied research in materials science, physics, chemistry and engineering in the area of plasma sources and plasma-based treatments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


