Live‐cell fluorescence imaging and optogenetic control of PKA kinase activity in fission yeast Schizosaccharomyces pombe
IF 2.6
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The cAMP‐PKA signaling pathway plays a crucial role in sensing and responding to nutrient availability in the fission yeast
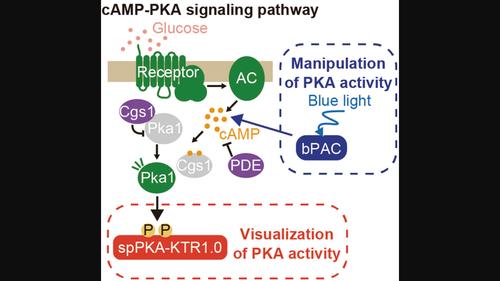
裂殖酵母中 PKA 激酶活性的活细胞荧光成像和光遗传控制
cAMP-PKA 信号通路在裂殖酵母(Schizosaccharomyces pombe)中感知和响应营养供应方面起着至关重要的作用。该通路监测外部葡萄糖水平,以控制细胞生长和性分化。然而,cAMP-PKA 通路响应外部刺激的时间动态仍不清楚,这主要是因为缺乏定量可视化该通路活性的工具。在这里,我们报告了基于激酶转位报告(KTR)的生物传感器 spPKA-KTR1.0 的开发情况,它使我们能够测量裂殖酵母细胞中 PKA 活性的动态。spPKA-KTR1.0 源自转录因子 Rst2,它在 PKA 激活时会从细胞核转位到细胞质。我们发现,spPKA-KTR1.0在细胞核和细胞质之间的转位是依赖于cAMP-PKA途径的,这表明spPKA-KTR1.0是裂殖酵母细胞中PKA活性的可靠指标。此外,我们还通过引入可光激活的腺苷酸环化酶 bPAC 和 spPKA-KTR1.0,建立了一个可同时观察和操纵 cAMP-PKA 信号动态的系统。该系统为以更高的时间分辨率研究裂殖酵母细胞中 cAMP-PKA 通路信号动态的作用提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Yeast
生物-生化与分子生物学
CiteScore
5.30
自引率
3.80%
发文量
55
审稿时长
3 months
期刊介绍:
Yeast publishes original articles and reviews on the most significant developments of research with unicellular fungi, including innovative methods of broad applicability. It is essential reading for those wishing to keep up to date with this rapidly moving field of yeast biology.
Topics covered include: biochemistry and molecular biology; biodiversity and taxonomy; biotechnology; cell and developmental biology; ecology and evolution; genetics and genomics; metabolism and physiology; pathobiology; synthetic and systems biology; tools and resources
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


