Synthesis, characterization, and evaluation of the CO2 adsorption capacities of beta, ZSM-12, ZSM-48, and silicalite-1 zeolites obtained in the presence of tetraethylammonium hydroxide and 1,6-diaminohexane as organic structure-directing agents
Abstract
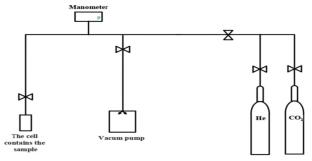
Zeolites synthesis using a combination of tetraethylammonium hydroxide (TEAOH) and 1,6-diaminohexane (DAH) as organic structure-directing agents (OSDAs) has been studied in order to understand how they acted during the synthesis. This combination can crystallize four zeolites: beta, ZSM-12, ZSM-48, and silicalite-1. TEAOH is found to direct the synthesis of beta, and ZSM-12 zeolites, whereas DAH directs the synthesis of ZSM-48 zeolite. On the other hand, the two organic components are found to have a cooperative role in the crystallization process of silicalite-1, with greater templating ability for TEAOH compared to DAH. The use of a supplementary organic component does not affect the degree of filling of the primary OSDA in the structure or the morphology of the obtained zeolites. The carbon dioxide (CO2) adsorption capacity of the zeolites obtained was evaluated at a temperature of 293 K in a pressure range of 0 to 1 atm. The relationship between the surface properties of the zeolites and their adsorption behavior was studied. Beta zeolite had the highest adsorption capacity with 2.9 mmol/g, while ZSM-48 zeolite had the lowest adsorption capacity with only 0.7 mmol/g.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


