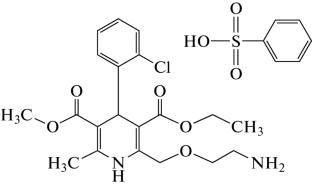
Solubility Study of Amlodipine Besylate in Ethylene Glycol + 2-Propanol Mixtures at Different Temperatures
IF 0.5
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Solubility behavior of amlodipine besylate (ADB) was investigated in the binary mixtures of ethylene glycol + 2-propanol at five different temperatures (293.2–313.2 K). The solubility results obtained from the shake-flask method are correlated with some reported cosolvency models (i.e. the van’t Hoff, combined nearly ideal binary solvent/Redlich–Kister, the Jouyban–Acree, the Jouyban–Acree–van’t Hoff, the mixture response surface, the modified Wilson, and Buchowski–Ksiazczak models). The accuracy of these models is investigated with the mean relative deviations of the back-calculated solubility data.
不同温度下苯磺酸氨氯地平在乙二醇和 2-丙醇混合物中的溶解度研究
摘要 研究了苯磺酸氨氯地平(ADB)在五种不同温度(293.2-313.2 K)下在乙二醇+2-丙醇二元混合物中的溶解行为。摇瓶法得出的溶解度结果与一些已报道的共溶模型(即 van't Hoff 模型、近乎理想的二元溶剂/Redlich-Kister 组合模型、Jouyban-Acree 模型、Jouyban-Acree-van't Hoff 模型、混合物响应面模型、改进的 Wilson 模型和 Buchowski-Ksiazczak 模型)相关联。这些模型的准确性是通过反向计算溶解度数据的平均相对偏差来研究的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Moscow University Chemistry Bulletin
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.30
自引率
14.30%
发文量
38
期刊介绍:
Moscow University Chemistry Bulletin is a journal that publishes review articles, original research articles, and short communications on various areas of basic and applied research in chemistry, including medical chemistry and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


