Validity and reliability study of a novel surface electromyography sensor using a well-consolidated electromyography system in individuals with cervical spinal cord injury
IF 2.1
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
Abstract
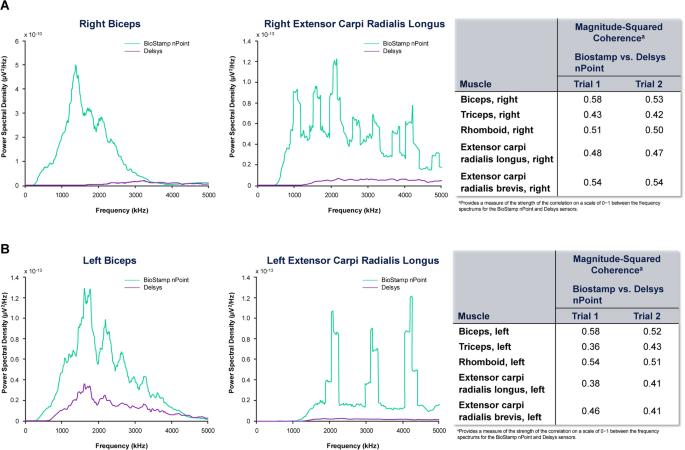
Non-interventional, cross-sectional pilot study. To establish the validity and reliability of the BioStamp nPoint biosensor (Medidata Solutions, New York, NY, USA [formerly MC10, Inc.]) for measuring electromyography in individuals with cervical spinal cord injury (SCI) by comparing the surface electromyography (sEMG) metrics with the Trigno wireless electromyography system (Delsys, Natick, MA, USA). Participants were recruited from the Shirley Ryan AbilityLab registry. Individuals aged 18–70 years with cervical SCI were evaluated with the two biosensors to capture activity on upper-extremity muscles during two study sessions conducted over 2 days (day 1–consent alone; day 2–two data collections in same session). Time and frequency metrics were captured, and signal-to-noise ratio was determined for each muscle group. Test-retest reliability was determined using Pearson’s correlation. Validation of the BioStamp nPoint system was based on Bland-Altmann analysis. Among the 11 participants, 30.8% had subacute cervical injury at C5–C6; 53.8% were injured within 1 year of the study. Results from the test-retest reliability assessment revealed that most Pearson’s correlations between the two sensory measurements were strong (≥0.50). The Bland-Altman analysis found values of the signal-to-noise ratio, frequency, and peak amplitude were within the level of agreement. Signal-to-noise ratios ranged from 7.06 to 22.1. In most instances, the performance of the BioStamp nPoint sensors was moderately to strongly correlated with that of the Trigno sensors in all muscle groups tested. The BioStamp nPoint system is a valid and reliable approach to assess sEMG measures in individuals with cervical SCI. The present study was supported by AbbVie Inc.
新型表面肌电图传感器的有效性和可靠性研究--在颈部脊髓损伤患者中使用固化良好的肌电图系统
通过比较表面肌电图 (sEMG) 指标与 Trigno 无线肌电图系统(Delsys,Natick,MA,USA),确定 BioStamp nPoint 生物传感器(Medidata Solutions,New York,NY,USA [前身为 MC10,Inc.])测量颈椎损伤 (SCI) 患者肌电图的有效性和可靠性。方法使用两种生物传感器对年龄在 18-70 岁的颈椎 SCI 患者进行评估,以捕捉他们在为期两天的两次研究过程中上肢肌肉的活动情况(第 1 天--仅征得同意;第 2 天--在同一研究过程中收集两次数据)。采集时间和频率指标,并确定每组肌肉的信噪比。使用皮尔逊相关性确定测试-重测可靠性。结果在 11 名参与者中,30.8% 在 C5-C6 处有亚急性颈椎损伤;53.8% 在研究后一年内受伤。重测可靠性评估结果显示,两种感觉测量之间的皮尔逊相关性大多很强(≥0.50)。布兰德-阿尔特曼分析发现,信噪比、频率和峰值振幅的值都在一致水平内。结论在大多数情况下,BioStamp nPoint 传感器的性能与 Trigno 传感器在所有测试肌肉群中的性能呈中度到高度相关。BioStamp nPoint 系统是评估颈椎 SCI 患者 sEMG 测量值的有效而可靠的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


