Dynamics of early-stage oxide formation on a Ni-Cr-Mo alloy
IF 6.6
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
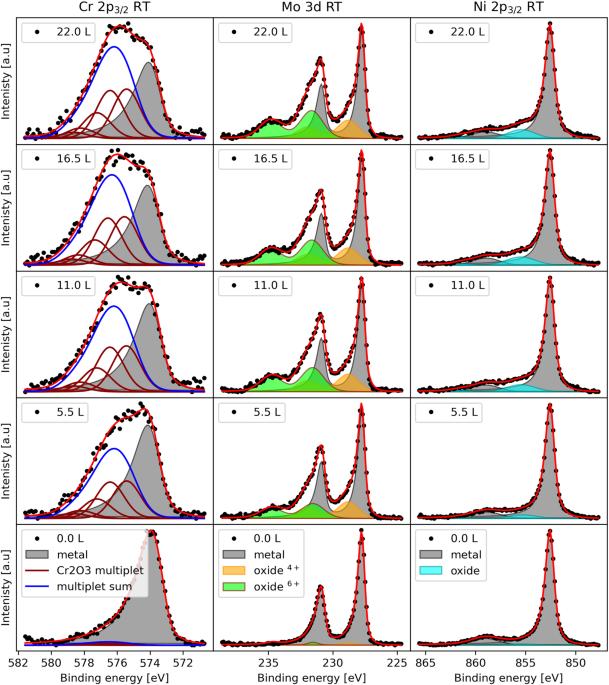
Corrosion results in large costs and environmental impact but can be controlled by thin oxide films that passivate the metal surfaces and hinder further oxidation or dissolution in an aqueous environment. The structure, chemistry, and thickness of these oxide films play a significant role in determining their anti-corrosion properties and the early-stage oxidation dynamics affect the properties of the developed oxide. Here, we use in situ X-ray Photoelectron Spectroscopy (XPS) to study the early-stage oxidation of a Ni-Cr-Mo alloy at room temperature and up to 400 °C. Cr and Mo begin to oxidize immediately after exposure to O2, and Cr3+, Mo4+, and Mo6+ oxides are formed. In contrast, Ni does not contribute significantly to the oxide film. A self-limiting oxide thickness, which did not depend on temperature below 400 °C, is observed. This is attributed to the consumption of available Cr and Mo near the surface, which results in an enrichment of metallic Ni under the oxide. The self-limited oxide thickness is 6–8 Å, which corresponds to 3–4 atomic layers of cations in the oxide. At 400 °C, sublimation of Mo6+ oxide is observed, resulting in the formation of an almost pure layer of Cr2O3 on the alloy surface. Lastly, a mechanism is presented that explains the formation of the bi-layer oxide structure observed for Ni-Cr-Mo alloys, which involves the enhanced migration of hexavalent Mo ions in the electric field, which drives mass transport during oxidation according to both the Cabrera Mott model and the Point Defect Model.
镍铬钼合金早期氧化物形成动力学
腐蚀会造成巨大的成本和环境影响,但可以通过钝化金属表面并阻碍其在水环境中进一步氧化或溶解的氧化物薄膜来加以控制。这些氧化物薄膜的结构、化学性质和厚度在决定其防腐蚀性能方面起着重要作用,而且早期氧化动态也会影响已形成的氧化物的性能。在此,我们使用原位 X 射线光电子能谱 (XPS) 研究了镍铬钼合金在室温和高达 400 °C 下的早期氧化过程。铬和钼在暴露于 O2 后立即开始氧化,并形成 Cr3+、Mo4+ 和 Mo6+ 氧化物。相比之下,镍对氧化膜的影响不大。在低于 400 °C 的温度下,可以观察到氧化物厚度的自我限制,这种限制与温度无关。这是由于表面附近的铬和钼消耗殆尽,导致金属镍在氧化物下富集。自限制氧化物厚度为 6-8 Å,相当于氧化物中阳离子的 3-4 个原子层。400 °C 时,观察到 Mo6+ 氧化物升华,从而在合金表面形成了几乎纯净的 Cr2O3 层。最后,我们提出了一种机制来解释 Ni-Cr-Mo 合金中观察到的双层氧化物结构的形成,该机制涉及六价 Mo 离子在电场中的迁移增强,根据 Cabrera Mott 模型和点缺陷模型,该机制在氧化过程中推动了质量传输。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

npj Materials Degradation
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
7.80
自引率
7.80%
发文量
86
审稿时长
6 weeks
期刊介绍:
npj Materials Degradation considers basic and applied research that explores all aspects of the degradation of metallic and non-metallic materials. The journal broadly defines ‘materials degradation’ as a reduction in the ability of a material to perform its task in-service as a result of environmental exposure.
The journal covers a broad range of topics including but not limited to:
-Degradation of metals, glasses, minerals, polymers, ceramics, cements and composites in natural and engineered environments, as a result of various stimuli
-Computational and experimental studies of degradation mechanisms and kinetics
-Characterization of degradation by traditional and emerging techniques
-New approaches and technologies for enhancing resistance to degradation
-Inspection and monitoring techniques for materials in-service, such as sensing technologies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


