Distinct members of the Caenorhabditis elegans CeMbio reference microbiota exert cryptic virulence that is masked by host defense
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Microbiotas are complex microbial communities that colonize specific niches in the host and provide essential organismal functions that are important in health and disease. Understanding the ability of each distinct community member to promote or impair host health, alone or in the context of the community, is imperative for understanding how differences in community structure affect host health and vice versa. Recently, a reference 12-member microbiota for the model organism Caenorhabditis elegans, known as CeMbio, was defined. Here, we show the differential ability of each CeMbio bacterial species to activate innate immunity through the conserved PMK-1/p38 MAPK, ACh-WNT, and HLH-30/TFEB pathways. Although distinct CeMbio members differed in their ability to activate the PMK-1/p38 pathway, the ability to do so did not correlate with bacterial-induced lifespan reduction in wild-type or immunodeficient animals. In contrast, most species activated HLH-30/TFEB and showed virulence toward hlh-30-deficient animals. These results suggest that the microbiota of C. elegans is rife with bacteria that can shorten the host's lifespan if host defense is compromised and that HLH-30/TFEB is a fundamental and key host protective factor.
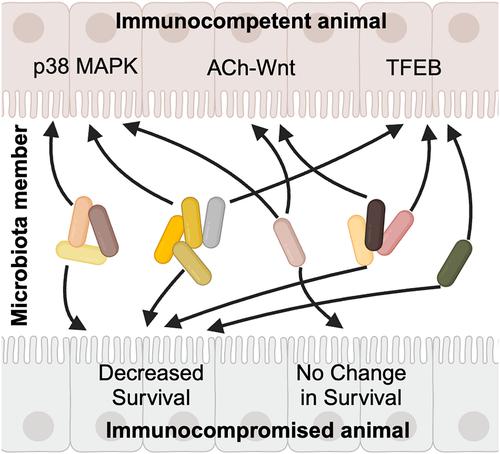
草履虫 CeMbio 参考微生物群的不同成员具有被宿主防御所掩盖的隐性毒力
微生物群落是一种复杂的微生物群落,它们在宿主体内的特定位置定殖,并提供对健康和疾病都很重要的基本生物功能。要了解群落结构的差异如何影响宿主健康,反之亦然,就必须了解每个不同群落成员单独或在群落背景下促进或损害宿主健康的能力。最近,为模式生物秀丽隐杆线虫(Caenorhabditis elegans)定义了一个由 12 个成员组成的参考微生物群(称为 CeMbio)。在这里,我们展示了每种 CeMbio 细菌通过保守的 PMK-1/p38 MAPK、ACh-WNT 和 HLH-30/TFEB 途径激活先天性免疫的不同能力。虽然不同的CeMbio成员激活PMK-1/p38通路的能力不同,但其能力与细菌诱导的野生型或免疫缺陷动物寿命缩短并无关联。相反,大多数物种都能激活 HLH-30/TFEB,并对 hlh-30 缺乏的动物表现出毒力。这些结果表明,秀丽隐杆线虫的微生物群中充斥着大量细菌,如果宿主的防御能力受损,这些细菌就会缩短宿主的寿命,而HLH-30/TFEB是宿主的一个基本和关键的保护因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


