Unexpected reaction of antimony pentafluoride with a fluorinated propellane
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
While 1,3-dehydro-5,7-difluoroadamantane 11 reacts with SbF5 in super acid media (SO2ClF) to generate the 1,3-dehydroadamant-5,7-diyl dication 1, 1,3-dehydro-5-fluoroadamantane 12 fails to react analogously to produce the 1,3-dehydroadamant-5-yl cation 9; instead, the 3,5-difluoro-1-adamantylcation 13 is formed. We present a computational study that explains this surprising behavior by the initial addition of Sb2F10 to the propellane bond present in 12. This is computed to be highly exothermic and leads to a zwitterionic (or ion-pair) species, which in turn reacts autocatalytically with HF to produce the observed fluorinated cation 13, SbF3, and SbF6–.
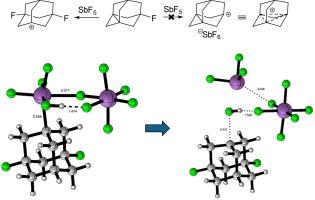
五氟化锑与含氟推进烷的意外反应
1,3-脱氢-5,7-二氟金刚烷 11 在超酸介质 (SO2ClF) 中与 SbF5 反应生成 1,3-脱氢金刚烷-5,7-二基二阳离子 1,而 1,3-脱氢-5-氟金刚烷 12 却未能发生类似反应生成 1,3-脱氢金刚烷-5-基阳离子 9;相反,生成了 3,5-二氟-1-金刚烷阳离子 13。我们提出了一项计算研究,通过在 12 中存在的丙烷键上添加 Sb2F10 来解释这种令人惊讶的行为。根据计算,这种加成反应具有很高的放热性,会产生一种齐聚物(或离子对),而这种齐聚物又会与 HF 自动催化反应,生成所观察到的氟化阳离子 13、SbF3 和 SbF6-。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


