Stabilization of layered lithium-rich manganese oxide for anion exchange membrane fuel cells and water electrolysers
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The design of materials that efficiently catalyse the electrochemical reaction of molecular oxygen to hydroxide ions is key to the development of electrochemical devices. Here we demonstrate an approach to control the orbital hybridization of 3d and 4d/5d metals to tune the adsorption strength and stabilize the catalytic sites in the platinum-free catalysts Li2Mn1−xRuxO3. We show that in these materials, the stabilization of O 2p holes by changing the M–O covalency (M = 4d/5d metal) can help to mitigate structural instability. Operando X-ray absorption spectroscopy revealed that the Mn and Ru atoms are the active sites for the oxygen reduction reaction (ORR) and exhibit a high ORR activity with noteworthy stability compared with the Pt/C catalyst and outperform NiFe layered double hydroxides and RuO2 in the oxygen evolution reaction. Notably, Li2Mn0.85Ru0.15O3 shows a high power density of 1.2 W cm−2 and current density of 1.2 A cm−2 at 1.9 V in the anion exchange membrane fuel cell and water electrolyser, respectively. The development of superior and cost-effective catalysts for the oxygen reduction and evolution reactions is pivotal for the future hydrogen economy. Now a series of Ru-modified Li2MnO3 catalysts have been designed to optimize the electronic structure and achieve a high performance in both oxygen reduction and evolution reactions, as demonstrated in practical anion exchange membrane fuel cell and water electrolyser tests.
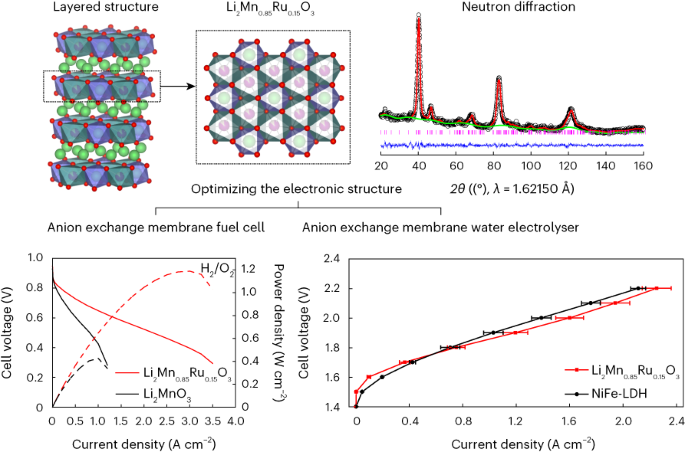
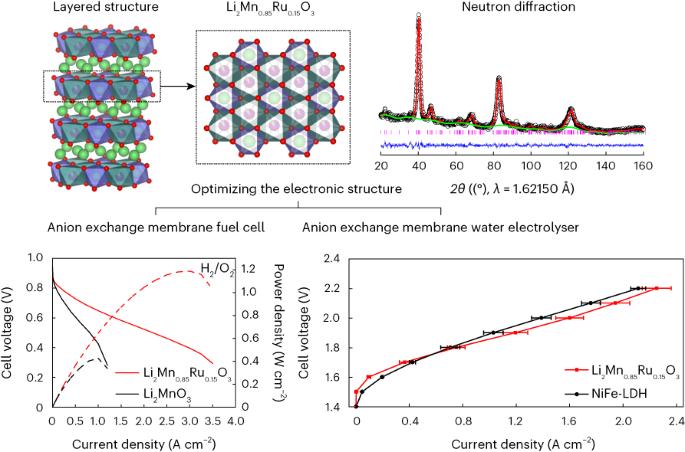
稳定用于阴离子交换膜燃料电池和水电解槽的层状富锂氧化锰
设计能有效催化分子氧到氢氧根离子电化学反应的材料是开发电化学设备的关键。在这里,我们展示了一种控制 3d 和 4d/5d 金属轨道杂化的方法,以调整无铂催化剂 Li2Mn1-xRuxO3 中的吸附强度并稳定催化位点。我们的研究表明,在这些材料中,通过改变 M-O 共价(M = 4d/5d 金属)来稳定 O 2p 孔有助于缓解结构的不稳定性。运算X射线吸收光谱显示,Mn和Ru原子是氧还原反应(ORR)的活性位点,与Pt/C催化剂相比,它们具有较高的ORR活性和显著的稳定性,在氧进化反应中的性能优于NiFe层状双氢氧化物和RuO2。值得注意的是,在阴离子交换膜燃料电池和水电解槽中,Li2Mn0.85Ru0.15O3 在 1.9 V 电压下的功率密度和电流密度分别高达 1.2 W cm-2 和 1.2 A cm-2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
文献相关原料
公司名称
产品信息
阿拉丁
MnCO3
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


