Mechanically triggered on-demand degradation of polymers synthesized by radical polymerizations
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Polymers that degrade on demand have the potential to facilitate chemical recycling, reduce environmental pollution and are useful in implant immolation, drug delivery or as adhesives that debond on demand. However, polymers made by radical polymerization, which feature all carbon-bond backbones and constitute the most important class of polymers, have proven difficult to render degradable. Here we report cyclobutene-based monomers that can be co-polymerized with conventional monomers and impart the resulting polymers with mechanically triggered degradability. The cyclobutene residues act as mechanophores and can undergo a mechanically triggered ring-opening reaction, which causes a rearrangement that renders the polymer chains cleavable by hydrolysis under basic conditions. These cyclobutene-based monomers are broadly applicable in free radical and controlled radical polymerizations, introduce functional groups into the backbone of polymers and allow the mechanically gated degradation of high-molecular-weight materials or cross-linked polymer networks into low-molecular-weight species. Radical polymerizations yield polymers that cannot easily be degraded. The co-polymerization of cyclobutene-based monomers with conventional vinyl monomers has now been shown to result in co-polymers with cyclobutane mechanophores in their backbone, which facilitate on-demand degradation through a combination of mechanical activation and hydrolysis. This approach offers a promising avenue for the degradation of all-carbon-bond-backbone polymers.
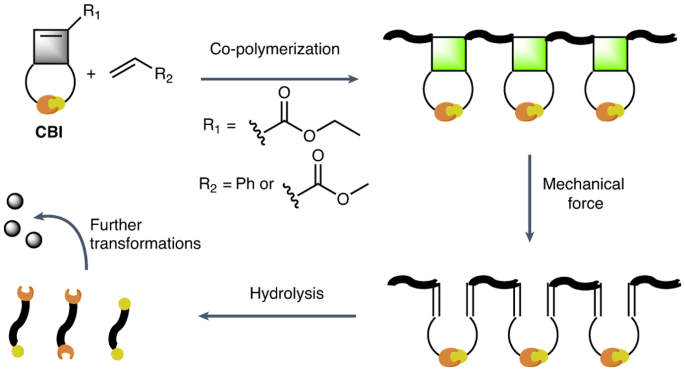
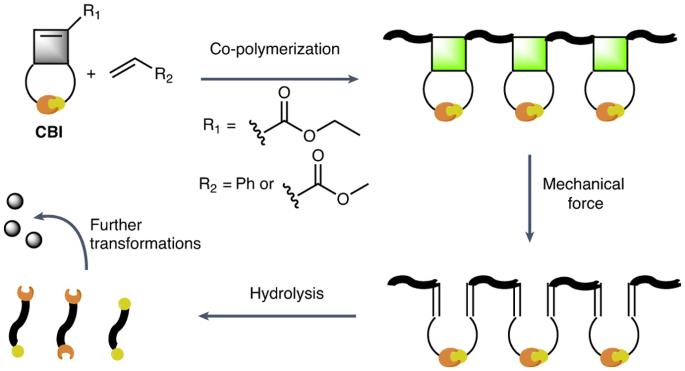
机械触发按需降解自由基聚合合成的聚合物
可按需降解的聚合物具有促进化学回收、减少环境污染的潜力,并可用于植入物固定、药物输送或用作按需脱落的粘合剂。然而,通过自由基聚合制成的聚合物具有全碳键骨架,是最重要的一类聚合物,但事实证明很难使其降解。在此,我们报告了可与传统单体共聚的环丁烯基单体,并赋予由此产生的聚合物机械触发降解性。环丁烯残基可作为机械分子,发生机械触发的开环反应,从而导致重排,使聚合物链在碱性条件下可通过水解裂解。这些环丁烯基单体可广泛应用于自由基和受控自由基聚合,在聚合物骨架中引入官能团,并可将高分子量材料或交联聚合物网络机械地降解为低分子量物质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


