Structure of the native γ-tubulin ring complex capping spindle microtubules
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Microtubule (MT) filaments, composed of α/β-tubulin dimers, are fundamental to cellular architecture, function and organismal development. They are nucleated from MT organizing centers by the evolutionarily conserved γ-tubulin ring complex (γTuRC). However, the molecular mechanism of nucleation remains elusive. Here we used cryo-electron tomography to determine the structure of the native γTuRC capping the minus end of a MT in the context of enriched budding yeast spindles. In our structure, γTuRC presents a ring of γ-tubulin subunits to seed nucleation of exclusively 13-protofilament MTs, adopting an active closed conformation to function as a perfect geometric template for MT nucleation. Our cryo-electron tomography reconstruction revealed that a coiled-coil protein staples the first row of α/β-tubulin of the MT to alternating positions along the γ-tubulin ring of γTuRC. This positioning of α/β-tubulin onto γTuRC suggests a role for the coiled-coil protein in augmenting γTuRC-mediated MT nucleation. Based on our results, we describe a molecular model for budding yeast γTuRC activation and MT nucleation. Using cryo-electron tomography, Dendooven et al. determined the structure of the native budding yeast γ-tubulin ring complex (γTuRC) capping spindle microtubules and showed that γTuRC adopts an active closed conformation to function as a perfect geometric template for microtubule nucleation.
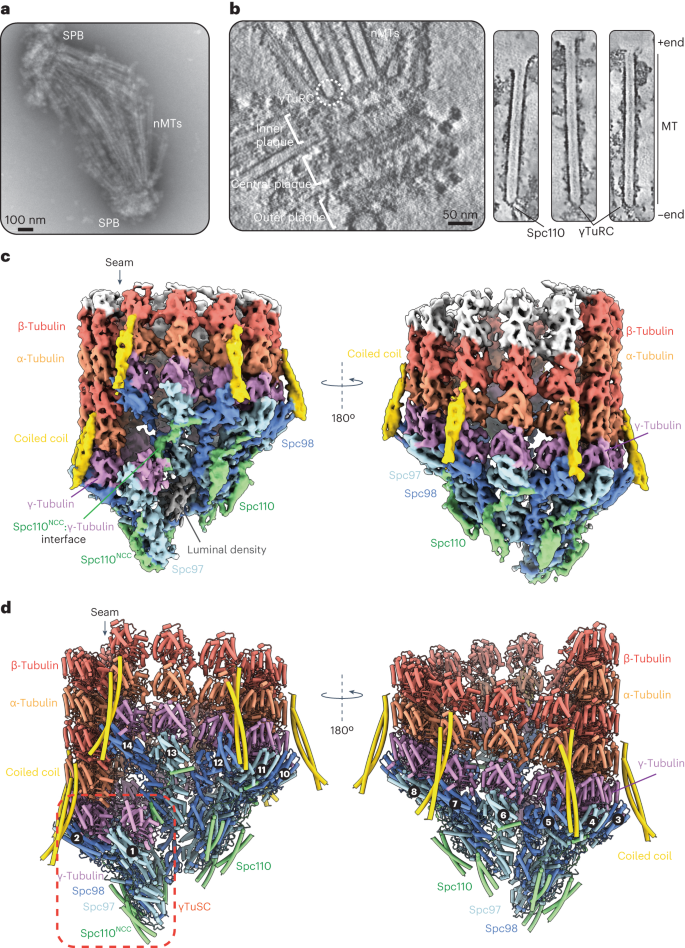
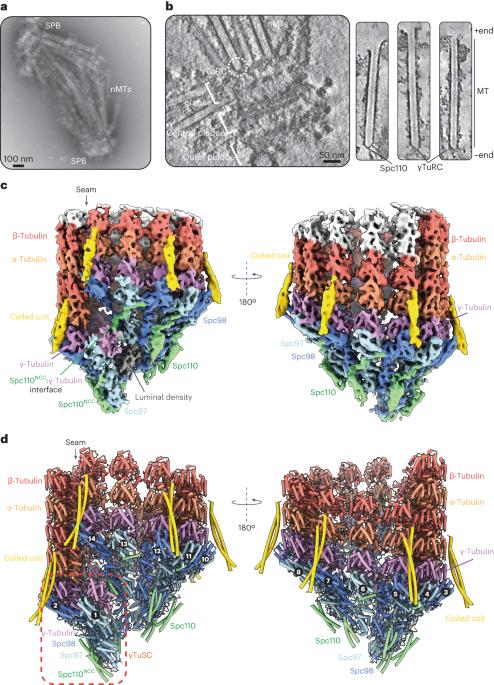
封闭纺锤体微管的原生γ-微管蛋白环状复合物的结构
微管(MT)丝由α/β-tubulin二聚体组成,是细胞结构、功能和生物体发育的基础。它们是通过进化保守的γ-微管蛋白环复合体(γTuRC)从MT组织中心成核的。然而,成核的分子机制仍然难以捉摸。在这里,我们利用低温电子断层扫描技术确定了在富集芽殖酵母纺锤体背景下封盖MT负端的原生γ-TuRC的结构。在我们的结构中,γTuRC呈现出一个由γ-tubulin亚基组成的环状结构,专门用于13-原丝MT的种子成核,它采用一种活跃的封闭构象,作为MT成核的完美几何模板。我们的低温电子断层扫描重建显示,一个盘绕线圈蛋白将MT的第一排α/β-tubulin钉在沿着γTuRC的γ-tubulin环的交替位置上。α/β-tubulin在γTuRC上的这种定位表明,盘绕蛋白在增强γTuRC介导的MT成核过程中发挥了作用。基于我们的研究结果,我们描述了芽殖酵母γTuRC活化和MT成核的分子模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


