Mild synthesis of 2,3,5,6-tetrafluoropyridine ethers and their reactivity toward imidazole and pyrazole
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A series of six, 4-substituted 2,3,5,6-tetrafluoropyridine (TFP) hetero ethers have been synthesized via regio-selective, nucleophilic aromatic substitution (SNAr) of pentafluoropyridine with aliphatic alcohols and phenols. Furthermore, the hexyloxy modified TFP substrate also served as a synthetic intermediate for the preparation of 3,5-difluoro-4-(hexyloxy)-2,6-di(1H-imidazol-1-yl)pyridine, as well as 4-(hexyloxy)-2,3,5,6-tetra(1H-pyrazol-1-yl)pyridine, which offers a terpyridine scaffold. This work outlines their synthetic methodology in addition to detailing the TFP intermediates’ unexpected reactivity with imidazole and pyrazole nucleophiles
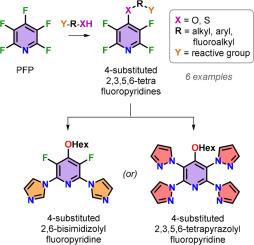
2,3,5,6-四氟吡啶醚的温和合成及其对咪唑和吡唑的反应活性
通过五氟吡啶与脂肪醇和苯酚的区域选择性亲核芳香取代(SNAr),合成了一系列六种 4 取代的 2,3,5,6-四氟吡啶(TFP)杂醚。此外,经己氧基修饰的 TFP 底物还是制备 3,5-二氟-4-(己氧基)-2,6-二(1H-咪唑-1-基)吡啶和 4-(己氧基)-2,3,5,6-四(1H-吡唑-1-基)吡啶的合成中间体,后者提供了一个三吡啶支架。除了详细介绍 TFP 中间体与咪唑和吡唑亲核物的意外反应性之外,这项研究还概述了它们的合成方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


