Joint-specific memory, resident memory T cells and the rolling window of opportunity in arthritis
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
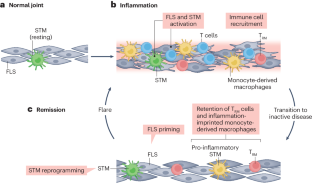
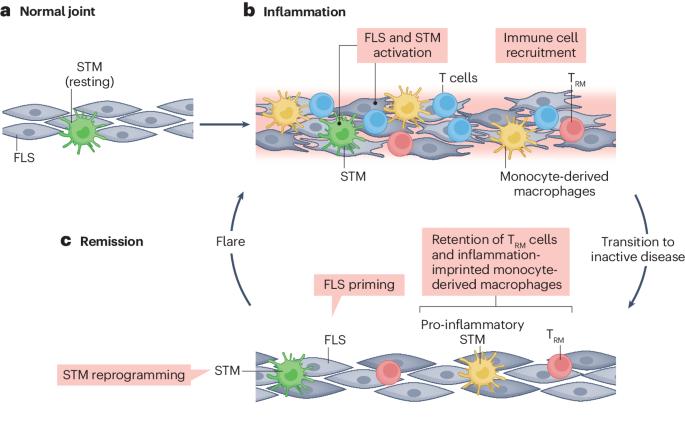
In rheumatoid arthritis, juvenile idiopathic arthritis and other forms of inflammatory arthritis, the immune system targets certain joints but not others. The pattern of joints affected varies by disease and by individual, with flares most commonly involving joints that were previously inflamed. This phenomenon, termed joint-specific memory, is difficult to explain by systemic immunity alone. Mechanisms of joint-specific memory include the involvement of synovial resident memory T cells that remain in the joint during remission and initiate localized disease recurrence. In addition, arthritis-induced durable changes in synovial fibroblasts and macrophages can amplify inflammation in a site-specific manner. Together with ongoing systemic processes that promote extension of arthritis to new joints, these local factors set the stage for a stepwise progression in disease severity, a paradigm for arthritis chronicity that we term the joint accumulation model. Although durable drug-free remission through early treatment remains elusive for most forms of arthritis, the joint accumulation paradigm defines new therapeutic targets, emphasizes the importance of sustained treatment to prevent disease extension to new joints, and identifies a rolling window of opportunity for altering the natural history of arthritis that extends well beyond the initiation phase of disease. This Review discusses joint-specific memory (the tendency of arthritis to recur in previously inflamed joints), explores the involvement of resident memory T cells and other contributors, and evaluates how arthritis might spread to new joints, emphasizing the important of sustained treatment.
关节特异性记忆、常驻记忆 T 细胞和关节炎的滚动机会之窗
在类风湿性关节炎、幼年特发性关节炎和其他形式的炎症性关节炎中,免疫系统的目标是某些关节,而不是其他关节。受影响关节的模式因疾病和个体而异,最常发作的是以前发炎的关节。这种现象被称为关节特异性记忆,很难单独用全身免疫来解释。关节特异性记忆的机制包括滑膜驻留记忆 T 细胞的参与,这些细胞在缓解期间留在关节内,并引发局部疾病复发。此外,关节炎引起的滑膜成纤维细胞和巨噬细胞的持久性变化也会以特定部位的方式扩大炎症。这些局部因素与促进关节炎扩展到新关节的持续系统过程一起,为疾病严重程度的逐步进展创造了条件,我们将这种关节炎慢性化模式称为关节积聚模式。虽然通过早期治疗实现持久的无药缓解对于大多数形式的关节炎来说仍然难以实现,但关节积聚模式定义了新的治疗目标,强调了持续治疗以防止疾病扩展到新关节的重要性,并确定了改变关节炎自然史的滚动机会之窗,它远远超出了疾病的起始阶段。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


