Paired photoelectrochemical conversion of CO2/H2O and glycerol at high rate
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photoelectrochemistry holds the promise of directly converting sunlight to valuable chemical products. Photoelectrochemical (PEC) methods, however, lag behind their electrochemical counterparts in terms of current density. In this work, we demonstrate that, by using concentrated sunlight, we can achieve current densities similar to electrochemical methods, but with lower energy input. Specifically, we combined the direct PEC oxidation of glycerol with the dark hydrogen evolution or CO2 reduction in a membrane-separated continuous-flow PEC cell. We achieved over 110 mA cm−2 photocurrent density, which is at least an order of magnitude larger than those typically reported in the literature. We demonstrated that the product distribution of glycerol oxidation is notably different in PEC and electrochemical scenarios at the same current density, and the parasitic oxygen evolution reaction can be suppressed in the PEC case. This approach raises opportunities to drive complex electrochemical reactions in a more selective manner. Photoelectrocatalysis offers the potential to reduce energy demand and provide different selectivity profiles compared with electrocatalytic analogues, but current systems have shown limited rates. Here, recent advances in light concentration and gas diffusion electrodes are integrated into a photoelectrochemical system for coupled glycerol oxidation and CO2/H2O reduction with photocurrent densities above 100 mA cm−2.
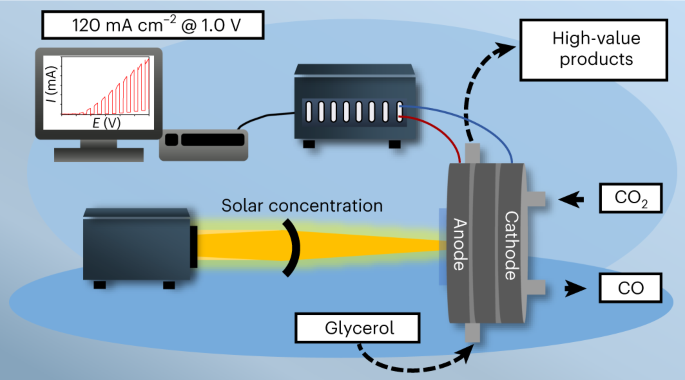
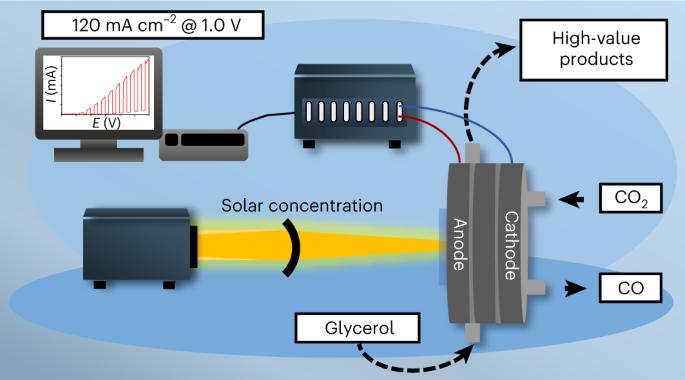
CO2/H2O 和甘油的配对光电化学高速转化
光电化学有望将太阳光直接转化为有价值的化学产品。然而,光电化学(PEC)方法在电流密度方面落后于电化学方法。在这项工作中,我们证明了通过利用集中的太阳光,我们可以获得与电化学方法相似的电流密度,但输入的能量更低。具体来说,我们将甘油的直接 PEC 氧化与暗氢气进化或二氧化碳还原结合在一个膜分离的连续流 PEC 电池中。我们获得了超过 110 mA cm-2 的光电流密度,比文献中通常报道的光电流密度至少大一个数量级。我们证明,在相同的电流密度下,PEC 和电化学方案中甘油氧化的产物分布明显不同,而且在 PEC 方案中可以抑制寄生氧进化反应。这种方法为以更具选择性的方式驱动复杂的电化学反应提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


