Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Bioisosteric replacement has emerged as a clear strategy for drug-structure optimization. Naphthalene is the core element of many chiral pharmaceuticals and drug candidates. However, as a promising isostere of naphthalene, the chiral version of 1,2-benzazaborine has rarely been explored due to the lack of efficient synthetic methods. Here we describe a copper-catalysed enantioselective hydroboration of alkenes with 1,2-benzazaborines. The method provides a general platform for the atom-economic and efficient construction of diverse chiral 1,2-benzazaborine compounds (more than 60 examples) that bear a 2-carbon-stereogenic centre or allene skeleton in high yields and excellent enantioselectivities. Three 1,2-benzazaborine analogues of bioactive chiral naphthalene-containing molecules have been prepared, and a series of transformations around chiral 1,2-benzazaborines have also been developed. Notably, the hydroboration process of this study reveals that the identity of 1,2-benzazaborine plays an essential role in the rate-determining step and catalyst resting state. Chiral 1,2-benzazaborines are promising isosteres of naphthalene, but rarely explored due to the lack of efficient synthetic methods. Now, the copper-catalysed enantioselective hydroboration of alkenes with 1,2-benzazaborines has been developed, providing a general platform for the atom-economic and efficient construction of diverse chiral 1,2-benzazaborine compounds bearing a 2-carbon-stereogenic centre or allene skeleton.
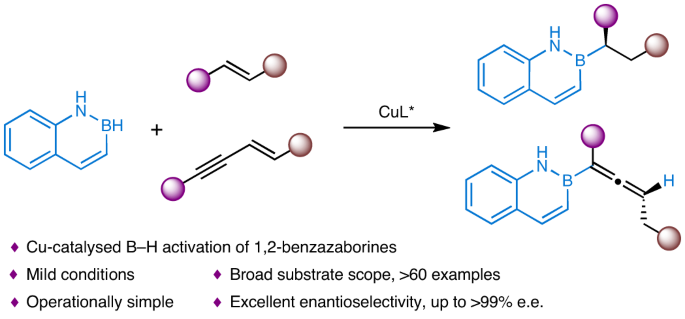
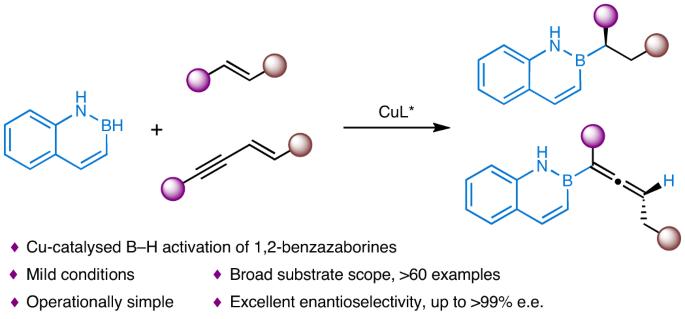
铜催化烯与 1,2-苯并氮硼烷的不对称氢硼化反应,以获得手性萘异构体
生物异构替代已成为药物结构优化的一种明确策略。萘是许多手性药物和候选药物的核心成分。然而,由于缺乏高效的合成方法,1,2-苯并氮硼烷作为萘的一种有前景的同系物,其手性版本很少被探索。在此,我们介绍了铜催化的 1,2-苯并氮硼烷与烯烃的对映选择性氢硼化合。该方法提供了一个通用平台,可以在原子经济的条件下高效地构建各种手性 1,2-苯并氮硼烷化合物(60 多个实例),这些化合物具有 2 碳立体中心或烯骨架,产量高且对映选择性极佳。目前已制备出三种具有生物活性的手性含萘分子的 1,2-苯并氮硼烷类似物,还开发出一系列围绕手性 1,2-苯并氮硼烷的转化方法。值得注意的是,本研究的氢硼化过程揭示了 1,2-苯并氮硼烷的特性在速率决定步骤和催化剂静止状态中起着至关重要的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


