Structure of adenylyl cyclase 5 in complex with Gβγ offers insights into ADCY5-related dyskinesia
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
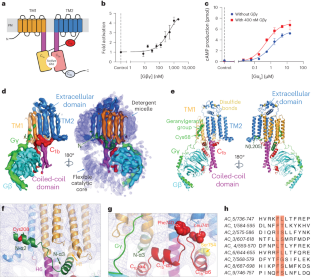
The nine different membrane-anchored adenylyl cyclase isoforms (AC1–9) in mammals are stimulated by the heterotrimeric G protein, Gαs, but their response to Gβγ regulation is isoform specific. In the present study, we report cryo-electron microscope structures of ligand-free AC5 in complex with Gβγ and a dimeric form of AC5 that could be involved in its regulation. Gβγ binds to a coiled-coil domain that links the AC transmembrane region to its catalytic core as well as to a region (C1b) that is known to be a hub for isoform-specific regulation. We confirmed the Gβγ interaction with both purified proteins and cell-based assays. Gain-of-function mutations in AC5 associated with human familial dyskinesia are located at the interface of AC5 with Gβγ and show reduced conditional activation by Gβγ, emphasizing the importance of the observed interaction for motor function in humans. We propose a molecular mechanism wherein Gβγ either prevents dimerization of AC5 or allosterically modulates the coiled-coil domain, and hence the catalytic core. As our mechanistic understanding of how individual AC isoforms are uniquely regulated is limited, studies such as this may provide new avenues for isoform-specific drug development. The authors describe the structure of an adenylyl cyclase 5 and Gβγ complex, which potentially influences a neural signalling pathway modulating motor function. Mutations in the Gβγ binding site on AC5 are linked to heritable forms of dyskinesia.

腺苷酸环化酶 5 与 Gβγ 复合物的结构为了解 ADCY5 相关运动障碍提供了思路
哺乳动物中九种不同的膜锚型腺苷酸环化酶同工型(AC1-9)受异源三聚体 G 蛋白 Gαs 的刺激,但它们对 Gβγ 调节的反应具有同工型特异性。在本研究中,我们报告了不含配体的 AC5 与 Gβγ 复合物的冷冻电镜结构,以及可能参与其调控的 AC5 的二聚体形式。Gβγ 与连接 AC 跨膜区和其催化核心的盘绕线圈结构域以及已知为异构体特异性调控枢纽的区域(C1b)结合。我们通过纯化的蛋白质和基于细胞的实验证实了 Gβγ 的相互作用。与人类家族性运动障碍相关的 AC5 功能增益突变位于 AC5 与 Gβγ 的交界处,并显示 Gβγ 的条件性激活减少,强调了所观察到的相互作用对人类运动功能的重要性。我们提出了一种分子机制,其中 Gβγ 要么阻止 AC5 的二聚化,要么异位调节盘绕线圈结构域,从而调节催化核心。由于我们对单个 AC 异构体如何受到独特调控的机理了解有限,类似的研究可能会为异构体特异性药物的开发提供新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


