Dual control over the reignition and combustion performance of hydroxylammonium nitrate-based gel propellants
IF 3.9
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
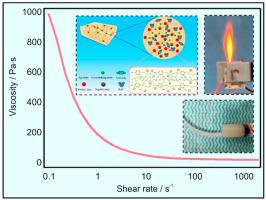
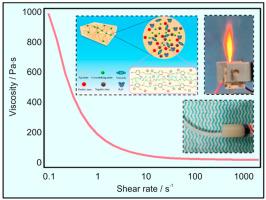
Hydroxylammonium nitrate (HAN; NH3OH + NO3−), a green and non-toxic monopropellant, finds wide application in liquid and controllable solid propulsion. Gel propulsion enjoys advantages such as a high throttling capacity and encouraging operational safety. This study prepared three HAN-based gel propellant samples with gelling agent contents ranging from 2 to 4 wt%. Their decomposition processes were analyzed using thermogravimetry (TG), differential scanning calorimetry (DSC), and mass spectrometry (MS), and a microthruster was designed to investigate their combustion characteristics under varying voltages and flow rates. Results reveal the presence of three exothermic peaks in the HAN-based gel propellants at temperatures of 204, 306 °C and 441 °C. The gel propellants experienced violent decomposition between 100 °C and 400 °C, producing low-molecular-weight organics such as C3H8, C2H6, H2O, O2, and NH3. For the initial ignition, the flow rate exerts a greater effect of reducing the delay time than the voltage. The reignition exhibited a shorter delay time than the initial ignition, and increasing voltage led to a more significant decrease in the reignition delay time than increasing the flow rate. Under a gelling agent content of approximately 4 wt%, the reignition delay time decreased from 2.35 s to 0.65 s as the voltage increased from 150 V to 250 V, with the flame length and light intensity during the reignition greater than those in the initial ignition. At the end of combustion, the extinguishment delay time changed insignificantly under high voltage. As revealed by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) analyses, residues with numerous cavities emerged due to incomplete combustion and the severe agglomeration of the gel propellants, exhibiting a maximum chlorine content of up to 77.73%.
对硝酸羟铵基凝胶推进剂的复燃和燃烧性能进行双重控制
硝酸羟铵(HAN;NH3OH + NO3-)是一种绿色无毒的单质推进剂,在液体和可控固体推进剂中应用广泛。凝胶推进剂具有节流能力强、操作安全等优点。本研究制备了三种基于 HAN 的凝胶推进剂样品,其胶凝剂含量为 2 至 4 wt%。使用热重仪(TG)、差示扫描量热仪(DSC)和质谱仪(MS)分析了它们的分解过程,并设计了一个微型推进器来研究它们在不同电压和流速下的燃烧特性。结果显示,HAN 基凝胶推进剂在 204、306 和 441 °C 温度下出现了三个放热峰。凝胶推进剂在 100 °C 至 400 °C 之间发生剧烈分解,产生 C3H8、C2H6、H2O、O2 和 NH3 等低分子量有机物。对于初始点火,流量比电压更能缩短延迟时间。复燃的延迟时间比初始点火的延迟时间短,增加电压比增加流速更能显著缩短复燃延迟时间。在胶凝剂含量约为 4 wt% 的条件下,当电压从 150 V 升至 250 V 时,复燃延迟时间从 2.35 s 缩短至 0.65 s,复燃时的火焰长度和光强均大于初始点火时的火焰长度和光强。燃烧结束时,熄灭延迟时间在高电压下变化不大。扫描电子显微镜(SEM)和能量色散光谱(EDS)分析表明,由于燃烧不完全和凝胶推进剂严重结块,残留物中出现了许多空穴,氯含量最高达 77.73%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energetic Materials Frontiers
Materials Science-Materials Science (miscellaneous)
CiteScore
6.90
自引率
0.00%
发文量
42
审稿时长
12 weeks
文献相关原料
公司名称
产品信息
希恩思
xanthan gum
阿拉丁
lithium perchlorate
|
99.0%
阿拉丁
lithium perchlorate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


