LC3B labeling of the parasitophorous vacuole membrane of Plasmodium berghei liver stage parasites depends on the V-ATPase and ATG16L1
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The protozoan parasite Plasmodium, the causative agent of malaria, undergoes an obligatory stage of intra-hepatic development before initiating a blood-stage infection. Productive invasion of hepatocytes involves the formation of a parasitophorous vacuole (PV) generated by the invagination of the host cell plasma membrane. Surrounded by the PV membrane (PVM), the parasite undergoes extensive replication. During intracellular development in the hepatocyte, the parasites provoke the Plasmodium-associated autophagy-related (PAAR) response. This is characterized by a long-lasting association of the autophagy marker protein, and ATG8 family member, LC3B with the PVM. LC3B localization at the PVM does not follow the canonical autophagy pathway since upstream events specific to canonical autophagy are dispensable. Here, we describe that LC3B localization at the PVM of Plasmodium parasites requires the V-ATPase and its interaction with ATG16L1. The WD40 domain of ATG16L1 is crucial for its recruitment to the PVM. Thus, we provide new mechanistic insight into the previously described PAAR response targeting Plasmodium liver stage parasites.
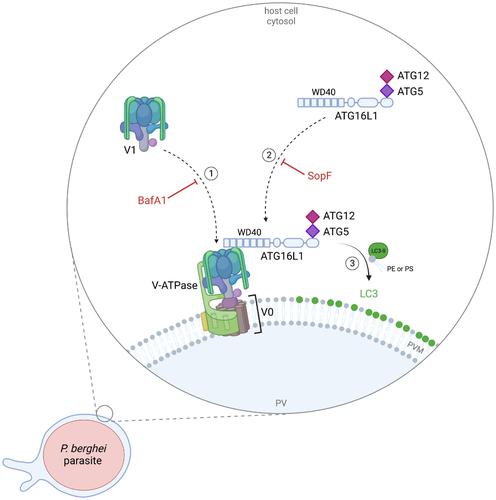
伯格希氏疟原虫肝期寄生虫寄生液泡膜的 LC3B 标记取决于 V-ATP 酶和 ATG16L1
原生动物疟原虫是疟疾的病原体,在开始血液阶段感染之前,必须经历一个肝内发育阶段。寄生虫侵入肝细胞后,宿主细胞质膜内陷,形成寄生虫泡(PV)。在空泡膜(PVM)的包围下,寄生虫进行广泛的复制。寄生虫在肝细胞内发育期间,会引发疟原虫相关自噬反应(PAAR)。这种反应的特点是自噬标记蛋白和 ATG8 家族成员 LC3B 与 PVM 长时间结合。LC3B 在 PVM 的定位并不遵循典型的自噬途径,因为典型自噬的上游特异事件是不可或缺的。在这里,我们描述了 LC3B 在疟原虫 PVM 的定位需要 V-ATP 酶及其与 ATG16L1 的相互作用。ATG16L1 的 WD40 结构域对其招募到 PVM 至关重要。因此,我们为之前描述的针对疟原虫肝阶段寄生虫的 PAAR 反应提供了新的机制认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


