RNAi-based drug design: considerations and future directions
IF 101.8
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
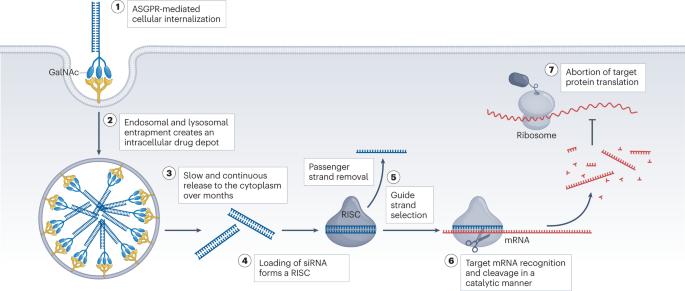
More than 25 years after its discovery, the post-transcriptional gene regulation mechanism termed RNAi is now transforming pharmaceutical development, proved by the recent FDA approval of multiple small interfering RNA (siRNA) drugs that target the liver. Synthetic siRNAs that trigger RNAi have the potential to specifically silence virtually any therapeutic target with unprecedented potency and durability. Bringing this innovative class of medicines to patients, however, has been riddled with substantial challenges, with delivery issues at the forefront. Several classes of siRNA drug are under clinical evaluation, but their utility in treating extrahepatic diseases remains limited, demanding continued innovation. In this Review, we discuss principal considerations and future directions in the design of therapeutic siRNAs, with a particular emphasis on chemistry, the application of informatics, delivery strategies and the importance of careful target selection, which together influence therapeutic success. Since the groundbreaking discovery of RNAi more than 25 years ago, several small interfering RNA (siRNA)-based therapies that target the liver have gained approval. This Review discusses principal considerations in siRNA-based drug development, focusing on the medicinal chemistry of siRNA design, the application of informatics, delivery platforms and future directions.
基于 RNAi 的药物设计:考虑因素和未来方向
被称为 RNAi 的转录后基因调控机制在被发现 25 年后的今天正在改变着医药开发,最近美国食品及药物管理局批准了多种针对肝脏的小干扰 RNA (siRNA) 药物就是证明。触发 RNAi 的合成 siRNA 有可能以前所未有的效力和持久性特异性地沉默几乎所有治疗靶点。然而,将这一类创新药物带给患者却面临着巨大的挑战,其中最突出的就是给药问题。有几类 siRNA 药物正在接受临床评估,但它们在治疗肝外疾病方面的作用仍然有限,需要不断创新。在这篇综述中,我们将讨论治疗用 siRNA 设计的主要考虑因素和未来方向,特别强调化学、信息学应用、给药策略和谨慎选择靶点的重要性,这些因素共同影响着治疗的成功与否。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


