Active Pd species in the formation of polysubstituted olefins and naphthalenes in the reaction between arylboronic acid and diphenylacetylene
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
‘Ligand-free’ Pd-catalyzed reaction between arylboronic acid and diphenylacetylene affords a set of polyphenylated olefins and 1,2,3,4-tetraphenylnaphthalene whose yields are dependent on counteranion of PdII salt and additive nature. Tetraphenylethylene and hexaphenylbuta-1,3-diene are likely formed in tandem arylation/cross-coupling reaction with the participation of hydroxo/alkoxo alkenyl Pd species, whereas 1,2,3,4-tetraphenylnaphthalene formation probably proceeds through tandem arylation/C–H activation by halide-containing alkenyl Pd complexes.
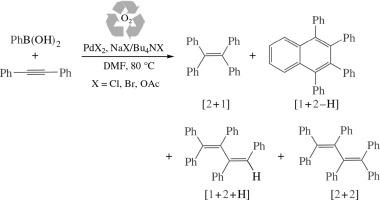
芳基硼酸与二苯基乙炔反应生成多取代烯烃和萘过程中的活性钯物种
芳基硼酸和二苯基乙炔在 "无配体 "Pd 催化下发生反应,生成一系列多苯基烯烃和 1,2,3,4 四苯基萘,其产率取决于 PdII 盐的反阴离子和添加剂的性质。四苯基乙烯和六苯基丁-1,3-二烯可能是在羟基/烷氧基烯基钯的参与下通过串联芳基化/交叉偶联反应形成的,而 1,2,3,4-四苯基萘的形成可能是通过含卤化物的烯基钯络合物的串联芳基化/C-H 活化进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


