Stereoselective synthesis of spirocyclic derivatives of functionalized 2,3,4,7-tetrahydro-1H-azepines
IF 1.8
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A simple and effective stereoselective synthesis of unique azaspirocyclic 2,3,4,7-tetrahydro-1H-azepine derivatives, namely, 7-azaspiro[4.6]undec-9-ene and 8-azaspiro[5.6]- dodec-10-ene, from simple commercially available reagents was accomplished. Their key 1,2-epoxy derivatives as individual diastereomers were obtained in nine steps with 31–32% overall yields. The possibility of synthesizing a library of small spiroheterocyclic molecules was exemplified by the N-Boc-protected (1RS,2SR,6RS)-1,2-epoxy-8-aza- spiro[5.6]dodec-10-ene scaffold through simple chemical modifications of its epoxide function.
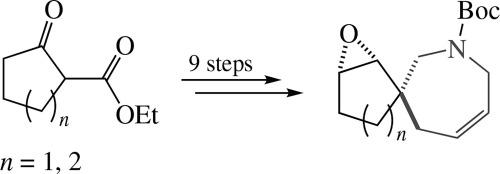
功能化 2,3,4,7-四氢-1H-氮杂卓螺环衍生物的立体选择性合成
本研究利用市售的简单试剂,简单有效地立体选择性合成了独特的氮杂环 2,3,4,7- 四氢-1H-氮杂卓衍生物,即 7- 氮杂螺[4.6]十一-9-烯和 8- 氮杂螺[5.6]十二-10-烯。通过九个步骤就得到了它们的关键 1,2-环氧衍生物的非对映异构体,总产率为 31-32%。通过对 N-叔丁氧羰基保护的 (1RS,2SR,6RS)-1,2-环氧-8-氮杂螺[5.6]十二-10-烯支架的环氧化物功能进行简单的化学修饰,合成了一个小型螺环化合物分子库。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


