Etrolizumab as an induction and maintenance therapy for ulcerative colitis: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background and Aim
Etrolizumab is a gut-targeted anti-β7 integrin monoclonal antibody. However, the evidence of etrolizumab efficacy and safety in ulcerative colitis remains inconclusive. Therefore, we aim to evaluate the safety and efficacy of etrolizumab as an induction and maintenance therapy for active moderate to severe ulcerative colitis.
Methods
We synthesized randomized controlled studies (RCTs) from MEDLINE, Scopus, EMBASE, PubMed, Web of Science, and Cochrane Library until April 2023. The risk ratio (RR) for dichotomous outcomes with the corresponding 95% confidence interval (CI) was used. The study protocol was registered in PROSPERO with ID: CRD42023437040.
Results
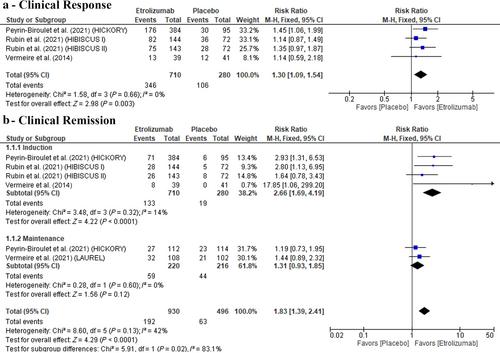
Five RCTs with 1849 participants were included. The etrolizumab group had a significant clinical response (RR: 1.28 with 95% CI [1.08, 1.51], P = 0.005), clinical remission rates during the induction phase (RR: 2.47 with 95% CI [1.48, 4.11], P = 0.0005), compared with the placebo group in ulcerative colitis; however, there was no statistically significant difference between the two groups, regarding the corticosteroids-free remission rate (RR: 1.92 with 95% CI [0.94, 3.92], P = 0.07). Moreover, endoscopic improvement, endoscopic remission, and histologic remission rates were observed more in the etrolizumab group during both the induction and maintenance phases. For safety outcomes, etrolizumab was significantly safer, but any adverse event was higher in the etrolizumab group than in the placebo.
Conclusion
Etrolizumab shows its effectiveness as both an induction and maintenance therapy for moderate or severe UC. The findings demonstrate its positive impact on clinical, endoscopic, and histologic remission rates. Regarding safety, other than any side effects, etrolizumab showed a good safety than a placebo.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


