Effect of a Declined Plasma Concentration of Valproic Acid Induced by Meropenem on the Antiepileptic Efficacy of Valproic Acid
Abstract
Objective
This study aimed to indicate whether a declined plasma concentration of valproic acid (VPA) induced by co-administration of meropenem (MEPM) could affect the antiepileptic efficacy of VPA.
Methods
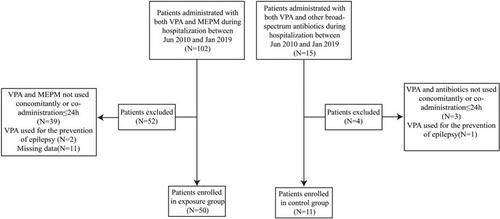
We retrospectively reviewed data of hospitalized patients who were diagnosed with status epilepticus or epilepsy between 2010 and 2019. Patients co-administered VPA and MEPM during hospitalization were screened and assigned to the exposure group, while those co-administerd VPA and other broad-spectrum antibiotics were allocated to the control group.
Results
The exposure group and control group included 50 and 11 patients, respectively. With a similar dosage of VPA, the plasma concentration of VPA significantly decreased during co-administration (24.6 ± 4.3 μg/mL) compared with that before co-administration (88.8 ± 13.6 μg/mL, p < 0.0001), and it was partly recovered with the termination of co-administration (39.8 ± 13.2 μg/mL, p = 0.163) in the exposure group. The inverse probability of treatment weighting estimated the treatment efficacy via changes in seizure frequency, seizure duration, and concomitant use of antiepileptic drugs, which were not significantly different between the exposure and control groups. In the exposure group, there was no significant differences in seizure frequency between the periods of before-during and before-after (p = 0.074 and 0.153, respectively). Seizure duration during VPA–MEPM co-administration was not significantly different from that before co-administration (p = 0.291).
Conclusions
In this study, the reduced plasma concentration of VPA induced by the co-administration of MEPM did not affect the antiepileptic efficacy of VPA. This conclusion should be interpreted with caution, and more research is warranted.
Trial Registration
Chinese Clinical Trial Registry: ChiCTR2000034567. Registered on 10 July 2020


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


