Cathepsin D inhibition during neuronal differentiation selectively affects individual proteins instead of overall protein turnover
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cathepsin D (CTSD) is a lysosomal aspartic protease and its inherited deficiency causes a severe pediatric neurodegenerative disease called neuronal ceroid lipofuscinosis (NCL) type 10. The lysosomal dysfunction in the affected patients leads to accumulation of undigested lysosomal cargo especially in none-dividing cells, such as neurons, resulting in death shortly after birth. To explore which proteins are mainly affected by the lysosomal dysfunction due to CTSD deficiency, Lund human mesencephalic (LUHMES) cells, capable of inducible dopaminergic neuronal differentiation, were treated with Pepstatin A. This inhibitor of “acidic” aspartic proteases caused accumulation of acidic intracellular vesicles in differentiating LUHMES cells. Pulse-chase experiments involving stable isotope labelling with amino acids in cell culture (SILAC) with subsequent mass-spectrometric protein identification and quantification were performed. By this approach, we studied the degradation and synthesis rates of 695 and 680 proteins during early and late neuronal LUHMES differentiation, respectively. Interestingly, lysosomal bulk proteolysis was not altered upon Pepstatin A treatment. Instead, the protease inhibitor selectively changed the turnover of individual proteins. Especially proteins belonging to the mitochondrial energy supply system were differentially degraded during early and late neuronal differentiation indicating a high energy demand as well as stress level in LUHMES cells treated with Pepstatin A.
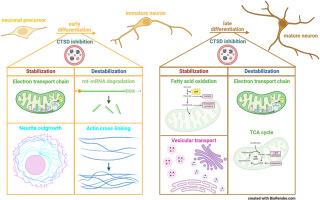
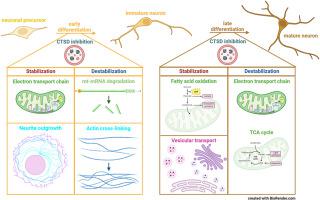
在神经元分化过程中抑制胰蛋白酶 D 会选择性地影响单个蛋白质,而不是整体蛋白质的周转。
溶酶体蛋白酶 D(CTSD)是一种溶酶体天冬氨酸蛋白酶,其遗传性缺乏会导致一种严重的儿科神经退行性疾病--神经细胞类脂质沉着病(NCL)10 型。受影响患者的溶酶体功能障碍会导致未消化的溶酶体货物堆积,尤其是在神经元等非分裂细胞中,从而导致患者出生后不久即死亡。为了探究CTSD缺乏症导致的溶酶体功能障碍主要影响哪些蛋白质,研究人员用Pepstatin A处理了能诱导多巴胺能神经元分化的伦德人间脑(LUHMES)细胞,这种 "酸性 "天冬氨酸蛋白酶抑制剂会导致分化中的LUHMES细胞内酸性囊泡的积累。我们进行了细胞培养中氨基酸稳定同位素标记(SILAC)的脉冲追逐实验,随后进行了质谱蛋白质鉴定和定量。通过这种方法,我们研究了神经元LUHMES分化早期和晚期分别有695和680种蛋白质的降解和合成率。有趣的是,Pepstatin A 处理并没有改变溶酶体的大量蛋白水解。相反,蛋白酶抑制剂选择性地改变了单个蛋白质的周转。特别是属于线粒体能量供应系统的蛋白质在神经元分化早期和晚期出现了不同程度的降解,这表明在使用 Pepstatin A 处理的 LUHMES 细胞中存在高能量需求和应激水平。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimie
生物-生化与分子生物学
CiteScore
7.20
自引率
2.60%
发文量
219
审稿时长
40 days
期刊介绍:
Biochimie publishes original research articles, short communications, review articles, graphical reviews, mini-reviews, and hypotheses in the broad areas of biology, including biochemistry, enzymology, molecular and cell biology, metabolic regulation, genetics, immunology, microbiology, structural biology, genomics, proteomics, and molecular mechanisms of disease. Biochimie publishes exclusively in English.
Articles are subject to peer review, and must satisfy the requirements of originality, high scientific integrity and general interest to a broad range of readers. Submissions that are judged to be of sound scientific and technical quality but do not fully satisfy the requirements for publication in Biochimie may benefit from a transfer service to a more suitable journal within the same subject area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


