Formulation and evaluation of multicomponent inclusion complex of cyclosporine A
Abstract
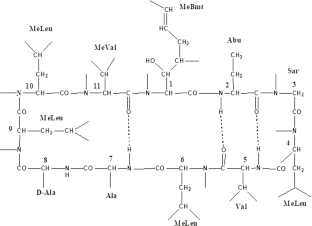
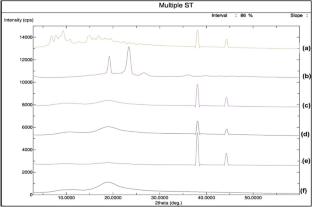
Cyclosporine A (CP) inclusion complex using cyclodextrin (binary) and cyclodextrin with TPGS (ternary) was prepared by the freeze-drying method. The phase solubility study was performed to calculate the solubility parameters. The prepared formulations were evaluated for saturation solubility and drug release studies. The spectroscopy and molecular docking studies were performed to confirm the formation of inclusion complex. The phase solubility results revealed a high stability constant for both binary and ternary samples. A significant enhancement in saturation solubility and dissolution was found in the prepared inclusion complexes. The spectroscopy studies revealed no interaction between the drug and carrier. The molecular docking study displayed the formation of a stable complex with a good docking score. The diffraction pattern showed the conversion of crystalline CP into an amorphous form after the formation of the inclusion complex. The findings were also supported by the saturation solubility study, which showed a significant enhancement in solubility. From the results, it can be concluded that Cyclosporine A inclusion complex using HP βCD with TPGS is an excellent delivery system. Therefore, the prepared delivery systems may be an alternative to the conventional delivery system for enhanced solubility of highly lipophilic drugs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


