Cryo-EM structures of amyloid-β and tau filaments in Down syndrome
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
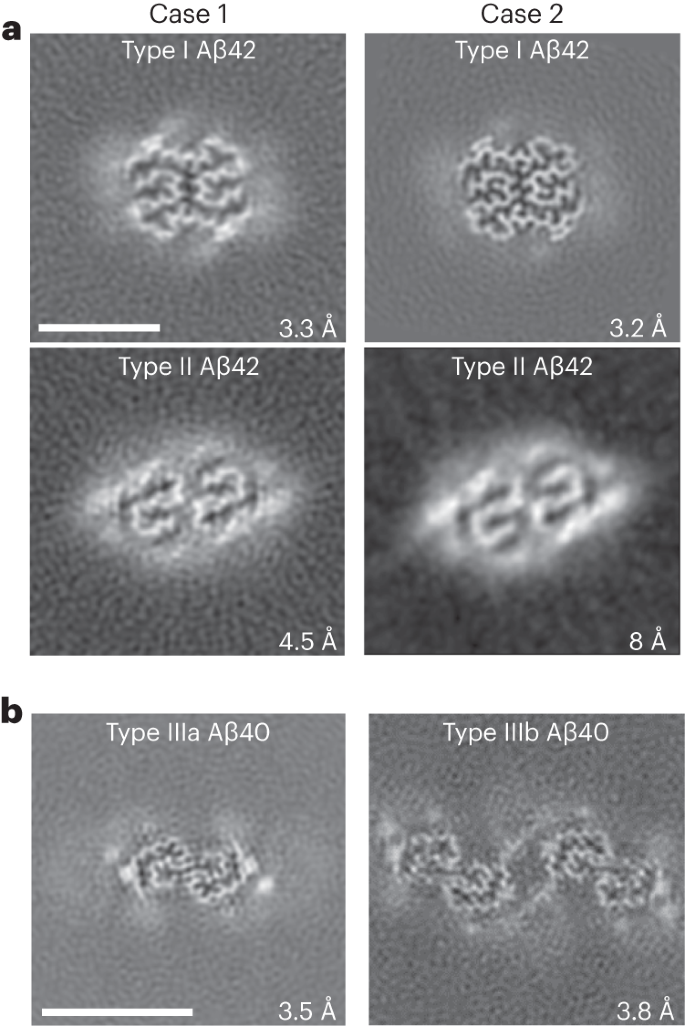
Adult individuals with Down syndrome (DS) develop Alzheimer disease (AD). Whether there is a difference between AD in DS and AD regarding the structure of amyloid-β (Aβ) and tau filaments is unknown. Here we report the structure of Aβ and tau filaments from two DS brains. We found two Aβ40 filaments (types IIIa and IIIb) that differ from those previously reported in sporadic AD and two types of Aβ42 filaments (I and II) identical to those found in sporadic and familial AD. Tau filaments (paired helical filaments and straight filaments) were identical to those in AD, supporting the notion of a common mechanism through which amyloids trigger aggregation of tau. This knowledge is important for understanding AD in DS and assessing whether adults with DS could be included in AD clinical trials. Here, using cryo-EM, authors reveal that amyloid-β and tau are identical in Alzheimer disease and Down syndrome. This has implications for assessing whether adults with Down syndrome could be included in Alzheimer disease clinical trials.
唐氏综合征中淀粉样蛋白-β和 tau 纤维的冷冻电镜结构
患有唐氏综合征(DS)的成年人会患上阿尔茨海默病(AD)。目前还不清楚唐氏综合征阿尔茨海默病和唐氏综合征阿尔茨海默病在淀粉样蛋白-β(Aβ)和 tau 纤维结构方面是否存在差异。在此,我们报告了两个 DS 大脑中 Aβ 和 tau 纤维的结构。我们发现了两种Aβ40细丝(IIIa型和IIIb型),它们不同于之前报道的散发性AD中的Aβ40细丝,也发现了两种类型的Aβ42细丝(I型和II型),它们与散发性和家族性AD中的Aβ42细丝相同。Tau丝(成对螺旋丝和直丝)与AD中的Tau丝相同,这支持了淀粉样蛋白触发tau聚集的共同机制这一观点。这些知识对于了解DS的注意力缺失症以及评估是否可将患有DS的成人纳入注意力缺失症临床试验非常重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


