Deletion of Dictyostelium tpc2 gene forms multi-tipped structures, regulates autophagy and cell-type patterning
Abstract
Background Information
Two pore channels (TPCs) are voltage-gated ion channel superfamily members that release Ca2+ from acidic intracellular stores and are ubiquitously present in both animals and plants. Starvation initiates multicellular development in Dictyostelium discoideum. Increased intracellular calcium levels bias Dictyostelium cells towards the stalk pathway and thus we decided to analyze the role of TPC2 in development, differentiation, and autophagy.
Results
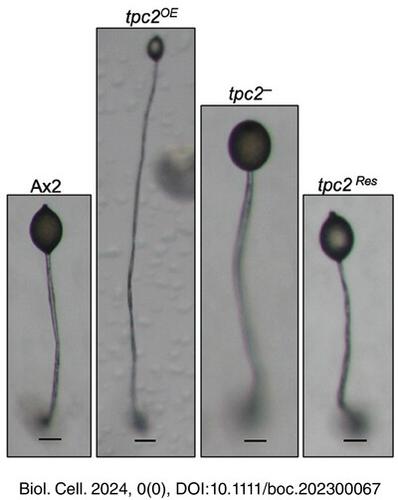
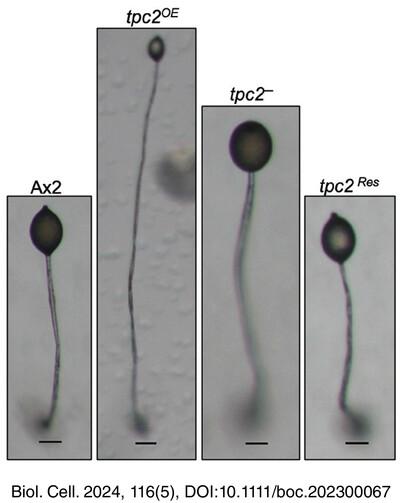
We showed TPC2 protein localizes in lysosome-like acidic vesicles and the in situ data showed stalk cell biasness. Deletion of tpc2 showed defective and delayed development with formation of multi-tipped structures attached to a common base, while tpc2OE cells showed faster development with numerous small-sized aggregates and wiry fruiting bodies. The tpc2OE cells showed higher intracellular cAMP levels as compared to the tpc2− cells while pinocytosis was found to be higher in the tpc2− cells. Also, TPC2 regulates cell-substrate adhesion and cellular morphology. Under nutrient starvation, deletion of tpc2 reduced autophagic flux as compared to Ax2. During chimera formation, tpc2− cells showed a bias towards the prestalk/stalk region while tpc2OE cells showed a bias towards the prespore/spore region. tpc2 deficient strain exhibits aberrant cell-type patterning and loss of distinct boundary between the prestalk/prespore regions.
Conclusion
TPC2 is required for effective development and differentiation in Dictyostelium and supports autophagic cell death and cell-type patterning.
Significance
Decreased calcium due to deletion of tpc2 inhibit autophagic flux.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


