TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer disease mouse models
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
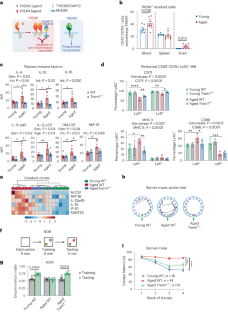
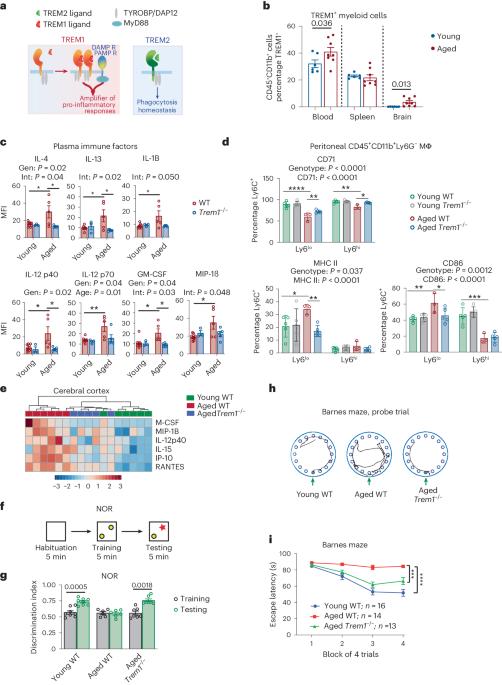
Human genetics implicate defective myeloid responses in the development of late-onset Alzheimer disease. A decline in peripheral and brain myeloid metabolism, triggering maladaptive immune responses, is a feature of aging. The role of TREM1, a pro-inflammatory factor, in neurodegenerative diseases is unclear. Here we show that Trem1 deficiency prevents age-dependent changes in myeloid metabolism, inflammation and hippocampal memory function in mice. Trem1 deficiency rescues age-associated declines in ribose 5-phosphate. In vitro, Trem1-deficient microglia are resistant to amyloid-β42 oligomer-induced bioenergetic changes, suggesting that amyloid-β42 oligomer stimulation disrupts homeostatic microglial metabolism and immune function via TREM1. In the 5XFAD mouse model, Trem1 haploinsufficiency prevents spatial memory loss, preserves homeostatic microglial morphology, and reduces neuritic dystrophy and changes in the disease-associated microglial transcriptomic signature. In aging APPSwe mice, Trem1 deficiency prevents hippocampal memory decline while restoring synaptic mitochondrial function and cerebral glucose uptake. In postmortem Alzheimer disease brain, TREM1 colocalizes with Iba1+ cells around amyloid plaques and its expression is associated with Alzheimer disease clinical and neuropathological severity. Our results suggest that TREM1 promotes cognitive decline in aging and in the context of amyloid pathology. The role of TREM1 in neurodegenerative diseases is unclear. Here the authors show that TREM1 promotes cognitive decline in aging and in the context of amyloid pathology in a mouse model of Alzheimer disease.
TREM1 在衰老和阿尔茨海默病小鼠模型中破坏髓系生物能和认知功能
人类遗传学认为,髓系反应缺陷与晚发性阿尔茨海默病的发病有关。外周和大脑髓系代谢下降,引发适应不良的免疫反应,是衰老的一个特征。TREM1是一种促炎因子,它在神经退行性疾病中的作用尚不清楚。我们在这里发现,Trem1 的缺乏可以防止小鼠髓系代谢、炎症和海马记忆功能发生年龄依赖性变化。Trem1的缺乏能挽救与年龄相关的核糖-5-磷酸的下降。在体外,Trem1缺陷的小胶质细胞对淀粉样蛋白-β42寡聚体诱导的生物能变化具有抵抗力,这表明淀粉样蛋白-β42寡聚体刺激通过TREM1破坏了小胶质细胞的平衡代谢和免疫功能。在 5XFAD 小鼠模型中,Trem1 单倍体缺失可防止空间记忆丧失,保护微胶质细胞的稳态形态,减少神经损伤和与疾病相关的微胶质细胞转录组特征的变化。在衰老的 APPSwe 小鼠中,Trem1 的缺乏可防止海马记忆衰退,同时恢复突触线粒体功能和脑葡萄糖摄取。在阿尔茨海默病死尸大脑中,TREM1与淀粉样斑块周围的Iba1+细胞共定位,其表达与阿尔茨海默病的临床和神经病理学严重程度相关。我们的研究结果表明,TREM1 在衰老和淀粉样病理的背景下会促进认知能力下降。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


