Low-frequency and rare genetic variants associated with rheumatoid arthritis risk
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
Rheumatoid arthritis (RA) has an estimated heritability of nearly 50%, which is particularly high in seropositive RA. HLA alleles account for a large proportion of this heritability, in addition to many common single-nucleotide polymorphisms with smaller individual effects. Low-frequency and rare variants, such as those captured by next-generation sequencing, can also have a large role in heritability in some individuals. Rare variant discovery has informed the development of drugs such as inhibitors of PCSK9 and Janus kinases. Some 34 low-frequency and rare variants are currently associated with RA risk. One variant (19:10352442G>C in TYK2) was identified in five separate studies, and might therefore represent a promising therapeutic target. Following a set of best practices in future studies, including studying diverse populations, using large sample sizes, validating RA and serostatus, replicating findings, adjusting for other variants and performing functional assessment, could help to ensure the relevance of identified variants. Exciting opportunities are now on the horizon for genetics in RA, including larger datasets and consortia, whole-genome sequencing and direct applications of findings in the management, and especially treatment, of RA. Rheumatoid arthritis has a substantial genetic component, some of which is associated with the presence of low-frequency or rare variants. Next-generation sequencing in large and well-defined cohorts can continue to identify these variants and thereby contribute to the future prediction, diagnosis and treatment of rheumatoid arthritis.
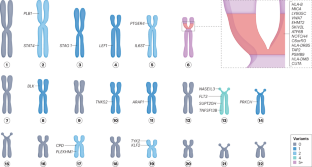
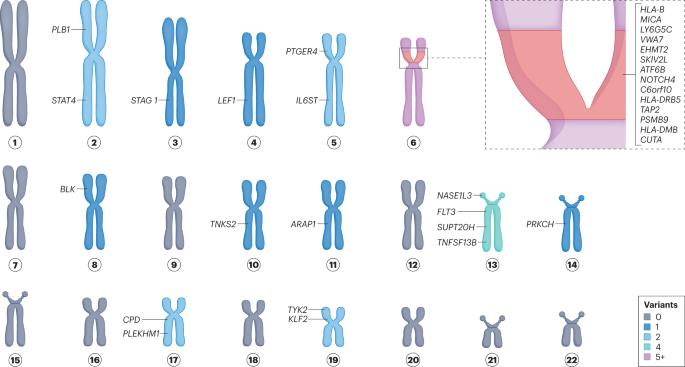
与类风湿性关节炎风险相关的低频和罕见基因变异
据估计,类风湿性关节炎(RA)的遗传率接近 50%,血清反应阳性的 RA 患者的遗传率尤其高。除了许多个体影响较小的常见单核苷酸多态性外,HLA等位基因也占遗传率的很大一部分。低频和罕见变异,如下一代测序所捕获的变异,在某些个体的遗传性中也能发挥重要作用。罕见变异的发现为 PCSK9 和 Janus 激酶抑制剂等药物的开发提供了依据。目前,约有 34 个低频罕见变异与 RA 风险有关。其中一个变体(TYK2中的19:10352442G>C)在五项单独的研究中被发现,因此可能是一个很有前景的治疗靶点。在未来的研究中,遵循一系列最佳实践,包括研究不同人群、使用大样本量、验证 RA 和血清状态、重复研究结果、调整其他变异以及进行功能评估,将有助于确保所发现变异的相关性。RA遗传学现在面临着令人兴奋的机遇,包括更大的数据集和联盟、全基因组测序以及将研究结果直接应用于RA的管理,尤其是治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


