Electrochemical hydrogenation and oxidation of organic species involving water
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Fossil fuel-driven thermochemical hydrogenation and oxidation using high-pressure H2 and O2 are still popular but energy-intensive CO2-emitting processes. At present, developing renewable energy-powered electrochemical technologies, especially those using clean, safe and easy-to-handle reducing agents and oxidants for organic hydrogenation and oxidation reactions, is urgently needed. Water is an ideal carrier of hydrogen and oxygen. Electrochemistry provides a powerful route to drive water splitting under ambient conditions. Thus, electrochemical hydrogenation and oxidation transformations involving water as the hydrogen source and oxidant, respectively, have been developed to be mild and efficient tools to synthesize organic hydrogenated and oxidized products. In this Review, we highlight the advances in water-participating electrochemical hydrogenation and oxidation reactions of representative organic molecules. Typical electrode materials, performance metrics and key characterization techniques are firstly introduced. General electrocatalyst design principles and controlling the microenvironment for promoting hydrogenation and oxygenation reactions involving water are summarized. Furthermore, paired hydrogenation and oxidation reactions are briefly introduced before finally discussing the challenges and future opportunities of this research field. The use of water for electrochemical hydrogenation and oxidation of organic species provides a sustainable route for synthesizing chemicals. The electrode types, general electrocatalyst selection principles and interface microenvironment control are elucidated, conducive to designing efficient electrocatalysts and reaction systems.
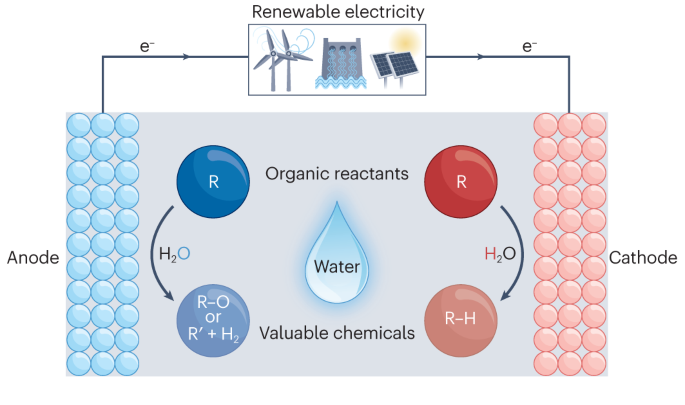
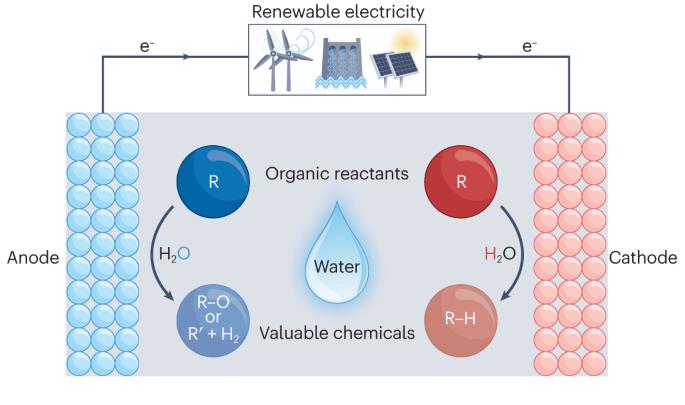
有机物的电化学氢化和水氧化。
使用高压 H2 和 O2 进行化石燃料驱动的热化学氢化和氧化反应仍然很流行,但却是高能耗的二氧化碳排放过程。目前,迫切需要开发以可再生能源为动力的电化学技术,特别是使用清洁、安全和易于处理的还原剂和氧化剂进行有机氢化和氧化反应的技术。水是氢和氧的理想载体。电化学为在环境条件下驱动水分裂提供了强有力的途径。因此,将水分别作为氢源和氧化剂的电化学氢化和氧化转化已发展成为合成有机氢化和氧化产物的温和而高效的工具。在本综述中,我们将重点介绍代表性有机分子的水参与电化学氢化和氧化反应的进展。首先介绍典型的电极材料、性能指标和关键表征技术。总结了促进水参与氢化和氧化反应的一般电催化剂设计原则和微环境控制。此外,还简要介绍了成对的氢化和氧化反应,最后讨论了这一研究领域面临的挑战和未来的机遇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


