Targeting the chromatin binding of exportin-1 disrupts NFAT and T cell activation
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Exportin-1 (XPO1/CRM1) plays a central role in the nuclear-to-cytoplasmic transport of hundreds of proteins and contributes to other cellular processes, such as centrosome duplication. Small molecules targeting XPO1 induce cytotoxicity, and selinexor was approved by the Food and Drug Administration in 2019 as a cancer chemotherapy for relapsed multiple myeloma. Here, we describe a cell-type-dependent chromatin-binding function for XPO1 that is essential for the chromatin occupancy of NFAT transcription factors and thus the appropriate activation of T cells. Additionally, we establish a class of XPO1-targeting small molecules capable of disrupting the chromatin binding of XPO1 without perturbing nuclear export or inducing cytotoxicity. This work defines a broad transcription regulatory role for XPO1 that is essential for T cell activation as well as a new class of XPO1 modulators to enable therapeutic targeting of XPO1 beyond oncology including in T cell-driven autoimmune disorders. Exportin-1 (XPO1) was identified as the target of small molecules suppressing T cell activation. Selective disruption of the chromatin scaffolding function of XPO1 without blocking nuclear export implicates XPO1 as a target in autoimmunity.
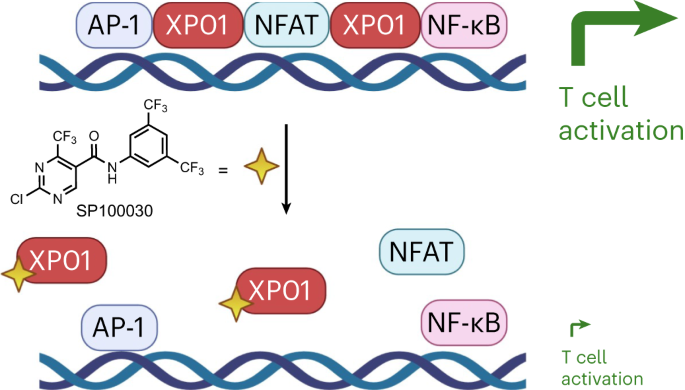
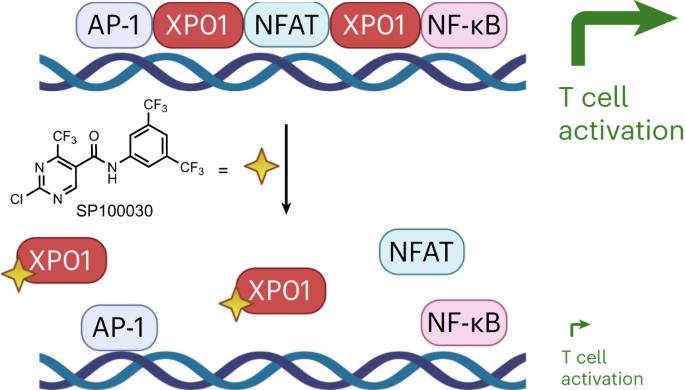
靶向 exportin-1 的染色质结合会破坏 NFAT 和 T 细胞的活化。
Exportin-1(XPO1/CRM1)在数百种蛋白质从细胞核到细胞质的转运过程中发挥着核心作用,并有助于中心体复制等其他细胞过程。靶向 XPO1 的小分子可诱导细胞毒性,2019 年,美国食品和药物管理局批准了 selinexor 作为复发多发性骨髓瘤的癌症化疗药物。在这里,我们描述了XPO1的一种细胞类型依赖性染色质结合功能,这种功能对于NFAT转录因子的染色质占据,从而适当激活T细胞至关重要。此外,我们还建立了一类 XPO1 靶向小分子,它们能够破坏 XPO1 的染色质结合,而不会扰乱核输出或诱导细胞毒性。这项工作为 XPO1 确定了一个广泛的转录调控角色,它对 T 细胞的活化以及一类新的 XPO1 调制剂至关重要,从而使 XPO1 的治疗靶点超越了肿瘤学,包括 T 细胞驱动的自身免疫性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


