Tissue-specific thresholds of mutation burden associated with anti-PD-1/L1 therapy benefit and prognosis in microsatellite-stable cancers
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 or its ligand (PD-1/L1) have expanded the treatment landscape against cancers but are effective in only a subset of patients. Tumor mutation burden (TMB) is postulated to be a generic determinant of ICI-dependent tumor rejection. Here we describe the association between TMB and survival outcomes among microsatellite-stable cancers in a real-world clinicogenomic cohort consisting of 70,698 patients distributed across 27 histologies. TMB was associated with survival benefit or detriment depending on tissue and treatment context, with eight cancer types demonstrating a specific association between TMB and improved outcomes upon treatment with anti-PD-1/L1 therapies. Survival benefits were noted over a broad range of TMB cutoffs across cancer types, and a dose-dependent relationship between TMB and outcomes was observed in a subset of cancers. These results have implications for the use of cancer-agnostic and universal TMB cutoffs to guide the use of anti-PD-1/L1 therapies, and they underline the importance of tissue context in the development of ICI biomarkers. Hsiehchen and colleagues assess the association between tumor mutational burden and survival in a real-world cohort of patients with microsatellite-stable cancers.

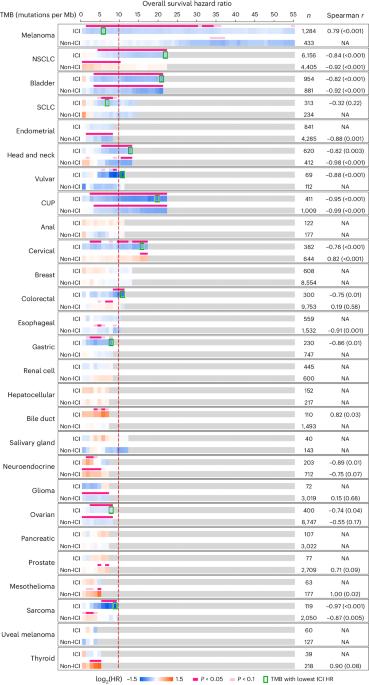
与微卫星稳定癌症中抗 PD-1/L1 治疗获益和预后相关的突变负荷组织特异性阈值。
以程序性细胞死亡蛋白1或其配体(PD-1/L1)为靶点的免疫检查点抑制剂(ICIs)扩大了癌症的治疗范围,但只对一部分患者有效。据推测,肿瘤突变负荷(TMB)是 ICI 依赖性肿瘤排斥反应的一般决定因素。在这里,我们描述了在一个真实世界的临床基因组队列中,TMB 与微卫星稳定癌症的生存结果之间的关系,该队列由 70,698 名患者组成,分布在 27 个组织学中。根据组织和治疗环境的不同,TMB 与生存获益或受损相关,其中有八种癌症类型显示出 TMB 与抗 PD-1/L1 疗法治疗后的预后改善之间存在特定的关联。在不同癌症类型中,TMB截断值的范围很宽,生存获益的范围也很广,而且在一部分癌症中观察到了TMB与疗效之间的剂量依赖关系。这些结果对使用癌症诊断和通用的TMB临界值来指导抗PD-1/L1疗法的使用具有重要意义,它们还强调了组织背景在开发ICI生物标记物中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


