Investigating the safety profiles of exogenous melatonin and associated adverse events: A pharmacovigilance study using WHO-VigiBase
Abstract
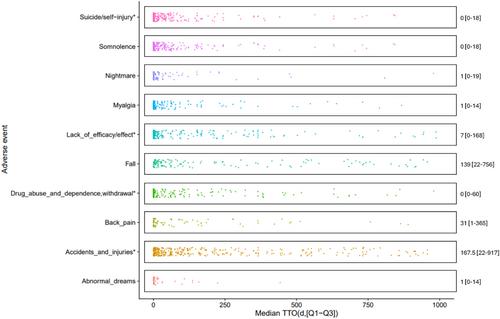
Melatonin, a pineal hormone that modulates circadian rhythms, sleep, and neurotransmitters, is widely used to treat sleep disorders. However, there are limited studies on the safety of melatonin. Therefore, we aimed to present the overall patterns of adverse events (AEs) following melatonin administration and identify potential safety signals associated with melatonin. Using VigiBase, a global individual case safety report (ICSRs) database managed by the World Health Organization (WHO), we conducted a retrospective, observational, pharmacovigilance study of melatonin between January 1996 and September 2022. Disproportionality analysis was conducted using two comparator settings: all other drugs and other sleep medications. We used multivariable logistic regression to estimate reporting odds ratios (RORs) with 95% confidence intervals (CIs) to compare the frequencies of AEs reporting between melatonin and each comparator setting. Furthermore, we assessed adverse events of special interests (AESIs) that could potentially be associated with melatonin. Signals were identified when the following criteria were met: cases ≥3, x2 ≥ 4, IC025 ≥ 0, and the lower end of the 95% CI of ROR > 2. These signals were then compared with the AE information on the drug labels provided by regulatory bodies. A total of 35 479 AE reports associated with melatonin were identified, with a higher proportion of reports from females (57.1%) and individuals aged 45–64 years (20.8%). We identified 21 AEs that were commonly detected as safety signals in the disproportionality analyses, including tic, educational problems, disturbance in social behavior, body temperature fluctuation, and growth retardation. In AESI analyses, accidents and injuries (adjusted ROR 2.97; 95% CI, 2.80–3.16), fall (2.24; 2.12–2.37), nightmare (4.90; 4.37–5.49), and abnormal dreams (3.68; 3.19–4.25) were detected as a signal of melatonin when compared to all other drugs, whereas those signals were not detected when compared to other sleep medications. In this pharmacovigilance study, exogenous melatonin showed safety profiles comparable to other sleep medications. However, several unexpected potential safety signals were identified, underscoring the need for further investigation at the population level.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


