Catalyst-free electrochemical SNAr of electron-rich fluoroarenes using carboxylic acids
IF 36.6
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Herein, an electrochemically driven catalyst-free nucleophilic aromatic substitution (SNAr) of electron-rich fluoroarenes with carboxylic acids as weak nucleophiles under mild conditions was reported. A series of highly valuable ester derivatives were obtained in a direct and rapid way. This transformation features commercially available reagents and an exceptionally broad substrate scope with good functional group tolerance, using cheap and abundant electrodes and completed within a short reaction time. Gram-scale synthesis and complex biorelevant compounds ligation further highlighted the potential utility of the methodology. The mechanistic investigations and density functional theory (DFT) calculations verified the feasibility of the proposed pathway of this transformation.
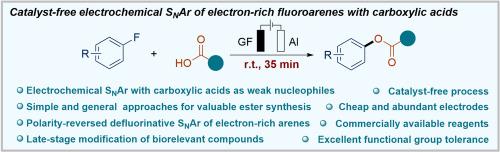
使用羧酸无催化剂电化学合成富电子芴的 SNAr
本文报告了在温和条件下,以羧酸为弱亲核体,通过电化学驱动无催化剂亲核芳香取代(SNAr)富电子氟烯烃的方法。研究人员以直接、快速的方式获得了一系列极具价值的酯类衍生物。这种转化方法的特点是试剂可在市场上买到,底物范围特别广,官能团耐受性好,使用的电极廉价而丰富,并能在很短的反应时间内完成。革兰氏级合成和复杂的生物相关化合物连接进一步凸显了该方法的潜在用途。机理研究和密度泛函理论(DFT)计算验证了所提出的转化途径的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


