A preliminary study of the mechanism of compound hearing loss caused by ototoxic drugs combined with impulse noise
Abstract
Background
Hearing loss (HL) is becoming increasingly common and is more commonly caused by noise, ototoxic substances, or a combination of ototoxic factors. However, so far, few studies have examined the mechanism by which compound factors cause HL. The only relevant study is about occupational ototoxic substances combined with environmental noise at 85–110 dB SPL. In this study, to address the shortcomings of existing research, we innovatively focused on HL induced by loud noise (impulse noise, >160 dB SPL) combined with common ototoxic drugs. The aim of this study was to establish and validate a mature animal model, and then to compare the characteristics of audiology, pathomorphology and molecular features, and to preliminarily predict pathogenesis in compound HL.
Materials and Methods
We selected guinea pigs to construct in vivo HL model groups for different extents of exposure, including a blank control group, a single-drug group, a single-impulse noise group, and a compound group. The animal model of the mature compound HL group was established using gentamicin combined with impulse noise. We then performed audiological and pathological verification. We analyzed the auditory brainstem response (ABR), pathological morphology of the cochlea, and molecules (including important self-radicals, cytokines, and apoptosis signal transduction pathway proteins in the pathogenesis of drug- and noise-induced HL), compared the effect of different extents of exposure on HL, and preliminarily predict the pathogenic mechanism of compound HL.
Results
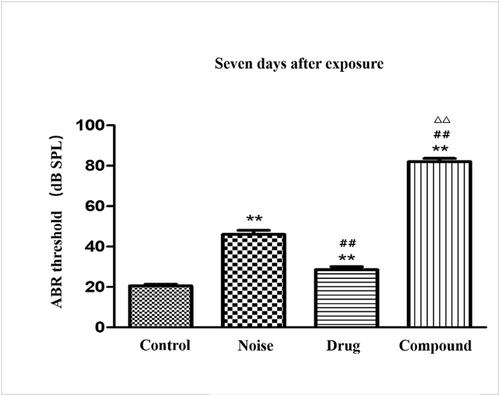
Four groups of animal models were established successfully and verified by audiology and pathology. Regarding audiology, there were no significant differences in the ABR thresholds before exposure (p > 0.05), but differences emerged among the groups after exposure. Notably, after 3, 7, and 14 days of exposure, there were significant differences in the ABR thresholds between the compound group and both the drug and noise groups (p < 0.01), and after 14 days, the HL of the compound group was much more severe (greater than the linear sum of single-factor HL group). Regarding the pathomorphology, compared with the control group, the cochleae were damaged to different degrees in the factor exposure groups. The drug group had the least severe HL, the noise group had serious HL (p < 0.05), and the compound group had the most severe HL (p < 0.01). The compound group's damage was greater than the linear sum of the single-factor group in many ways, such as the loss and damage of hair cells and cilia, disturbed morphology and arrangement of hair cells, protein metabolism, cell function, and structural defects on the epidermal plate (p < 0.01). From a molecular perspective, the trend was similar to pathology and audiology, and the synergistic effect of ototoxic drugs and impulse noise significantly increased cytokine levels (IL-6, ICAM-1,8-OHDG, IL-1, and TNF-α), free radicals Malondialdehyde ([MDA], ▪OH, LPO, O•2ˉ), and the apoptosis signal transduction pathway protein. There were significant differences between the compound group and single-factor groups (p < 0.05).
Conclusion
Gentamicin, impulse noise, and compound factors were used to induce HL in animal models, which were verified by audiology and pathology, laying a foundation for future studies. After constructing the animal models, we found that 50 mg/kg of gentamicin for 10 days was a subinjury dose, and 50× impulse noise caused partial HL, but the two factors combined had a significant synergistic ototoxicity effect, which increased the level of oxidative stress and the waterfall response of inflammatory cytokines in the cochleae and enhanced the expression of apoptosis-related proteins, resulting in synergistic pathomorphological and audiological injury. We preliminarily analyzed the pathogenic mechanism of compound HL, establishing the basis for further study of the mechanism, prevention, and treatment of this increasing global problem.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


