Kurarinone regulates Th17/Treg balance and ameliorates autoimmune uveitis via Rac1 inhibition
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Autoimmune uveitis (AU) is a severe intraocular autoimmune disorder with a chronic disease course and a high rate of blindness. Kurarinone (KU), a major component of the traditional Chinese medicine Sophorae Flavescentis Radix, possesses a wide spectrum of activities and has been used to treat several inflammation-related diseases.
Objective
We aimed to investigate the effects of KU on AU and its modulatory mechanisms.
Methods
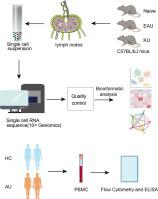
We used an experimental autoimmune uveitis (EAU) animal model and characterized the comprehensive immune landscape of KU-treated EAU mice using single-cell RNA sequencing (scRNA-seq). The retina and lymph nodes were analyzed. The siRNAs and selective inhibitors were used to study the signaling pathway. The effect of KU on peripheral blood mononuclear cells (PBMCs) from uveitis patients was also examined.
Results
We found that KU relieved chorioretinal lesions and immune cell infiltration in EAU model mice. Subsequent single-cell analysis revealed that KU downregulated the EAU-upregulated expression of inflammatory and autoimmune-related genes and suppressed pathways associated with immune cell differentiation, activation, and migration in a cell-specific manner. KU was implicated in restoring T helper 17 (Th17)/regulatory T (Treg) cell balance by alleviating inflammatory injury and elevating the expression of modulatory mediators in Tregs, while simultaneously ameliorating excessive inflammation by Th17 cells. Furthermore, Rac1 and the Id2/Pim1 axis potentiated the pathogenicity of Th17 cells during EAU, which was inhibited by KU treatment, contributing to the amelioration of EAU-induced inflammation and treatment of AU. In addition, KU suppressed inflammatory cytokine production in activated human PBMCs by inhibiting Rac1. Integration of the glucocorticoid-treated transcriptome suggests that KU has immunomodulatory effects on lymphocytes.
Conclusion
Our study constructed a high-resolution atlas of the immunoregulatory effects of KU treatment on EAU and identified its potential therapeutic mechanisms, which hold great promise in treating autoimmune disorders.

库拉灵酮通过抑制Rac1调节Th17/Treg平衡并改善自身免疫性葡萄膜炎
简介自身免疫性葡萄膜炎(AU)是一种严重的眼内自身免疫性疾病,病程慢性,致盲率高。Kurarinone (KU)是中药槐花的主要成分,具有广泛的活性,已被用于治疗多种炎症相关疾病:我们旨在研究 KU 对 AU 的影响及其调节机制:方法:我们使用实验性自身免疫性葡萄膜炎(EAU)动物模型,并使用单细胞RNA测序(scRNA-seq)表征了KU处理的EAU小鼠的综合免疫格局。对视网膜和淋巴结进行了分析。使用 siRNAs 和选择性抑制剂研究信号通路。我们还研究了 KU 对葡萄膜炎患者外周血单核细胞(PBMCs)的影响:结果:我们发现 KU 能缓解 EAU 模型小鼠的脉络膜视网膜病变和免疫细胞浸润。随后的单细胞分析表明,KU 下调了 EAU 上调的炎症和自身免疫相关基因的表达,并以细胞特异性的方式抑制了与免疫细胞分化、活化和迁移相关的通路。KU通过减轻炎症损伤和提高Tregs中调节介质的表达,同时改善Th17细胞的过度炎症,从而恢复T辅助细胞17(Th17)/调节性T(Treg)细胞的平衡。此外,Rac1和Id2/Pim1轴在EAU期间增强了Th17细胞的致病性,而KU治疗抑制了这种致病性,从而有助于改善EAU诱导的炎症和治疗AU。此外,KU 还能通过抑制 Rac1 抑制活化的人 PBMCs 中炎性细胞因子的产生。整合糖皮质激素处理的转录组表明,KU对淋巴细胞具有免疫调节作用:我们的研究构建了KU治疗对EAU免疫调节作用的高分辨率图谱,并确定了其潜在的治疗机制,这为治疗自身免疫性疾病带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


