Acidic/hypoxia dual-alleviated nanoregulators for enhanced treatment of tumor chemo-immunotherapy
Abstract
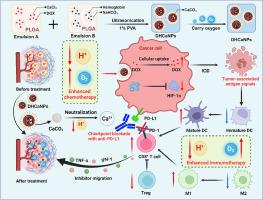
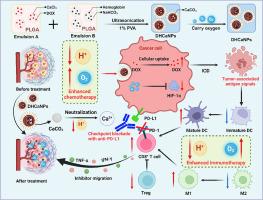
Chemotherapy plays a crucial role in triple-negative breast cancer (TNBC) treatment as it not only directly kills cancer cells but also induces immunogenic cell death. However, the chemotherapeutic efficacy was strongly restricted by the acidic and hypoxic tumor environment. Herein, we have successfully formulated PLGA-based nanoparticles concurrently loaded with doxorubicin (DOX), hemoglobin (Hb) and CaCO3 by a CaCO3-assisted emulsion method, aiming at the effective treatment of TNBC. We found that the obtained nanomedicine (DHCaNPs) exhibited effective drug encapsulation and pH-responsive drug release behavior. Moreover, DHCaNPs demonstrated robust capabilities in neutralizing protons and oxygen transport. Consequently, DHCaNPs could not only serve as oxygen nanoshuttles to attenuate tumor hypoxia but also neutralize the acidic tumor microenvironment (TME) by depleting lactic acid, thereby effectively overcoming the resistance to chemotherapy. Furthermore, DHCaNPs demonstrated a notable ability to enhance antitumor immune responses by increasing the frequency of tumor-infiltrating effector lymphocytes and reducing the frequency of various immune-suppressive cells, therefore exhibiting a superior efficacy in suppressing tumor growth and metastasis when combined with anti-PD-L1 (αPD-L1) immunotherapy. In summary, this study highlights that DHCaNPs could effectively attenuate the acidic and hypoxic TME, offering a promising strategy to figure out an enhanced chemo-immunotherapy to benefit TNBC patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


