Threonine fuels glioblastoma through YRDC-mediated codon-biased translational reprogramming
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
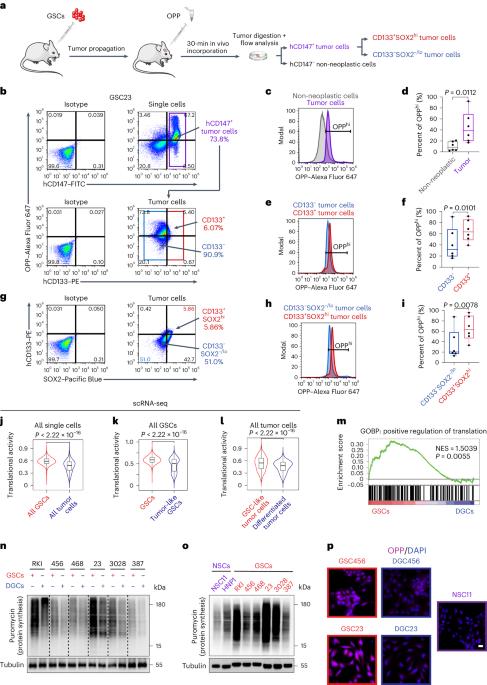
Cancers commonly reprogram translation and metabolism, but little is known about how these two features coordinate in cancer stem cells. Here we show that glioblastoma stem cells (GSCs) display elevated protein translation. To dissect underlying mechanisms, we performed a CRISPR screen and identified YRDC as the top essential transfer RNA (tRNA) modification enzyme in GSCs. YRDC catalyzes the formation of N6-threonylcarbamoyladenosine (t6A) on ANN-decoding tRNA species (A denotes adenosine, and N denotes any nucleotide). Targeting YRDC reduced t6A formation, suppressed global translation and inhibited tumor growth both in vitro and in vivo. Threonine is an essential substrate of YRDC. Threonine accumulated in GSCs, which facilitated t6A formation through YRDC and shifted the proteome to support mitosis-related genes with ANN codon bias. Dietary threonine restriction (TR) reduced tumor t6A formation, slowed xenograft growth and augmented anti-tumor efficacy of chemotherapy and anti-mitotic therapy, providing a molecular basis for a dietary intervention in cancer treatment. Rich and colleagues show that glioblastoma stem cells have increased global protein translation, which is achieved via the tRNA modifier YRDC. They show that targeting it or reducing its substrate threonine suppresses tumor growth.
苏氨酸通过 YRDC 介导的密码子偏向翻译重编程为胶质母细胞瘤提供燃料。
癌症通常会对翻译和新陈代谢进行重编程,但人们对癌症干细胞如何协调这两个特征知之甚少。在这里,我们发现胶质母细胞瘤干细胞(GSCs)显示出蛋白质翻译的升高。为了剖析其潜在机制,我们进行了一次CRISPR筛选,发现YRDC是GSCs中最重要的转运RNA(tRNA)修饰酶。YRDC 催化 ANN 解码 tRNA 上 N6-苏氨酰氨基甲酰基腺苷(t6A)的形成(A 表示腺苷,N 表示任何核苷酸)。以 YRDC 为靶标可减少 t6A 的形成,抑制全局翻译,并在体外和体内抑制肿瘤生长。苏氨酸是 YRDC 的重要底物。苏氨酸在 GSC 中积累,通过 YRDC 促进了 t6A 的形成,并使蛋白质组转向支持有丝分裂相关基因的 ANN 密码子偏倚。膳食苏氨酸限制(TR)减少了肿瘤t6A的形成,减缓了异种移植的生长,增强了化疗和抗有丝分裂疗法的抗肿瘤疗效,为膳食干预癌症治疗提供了分子基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


