Oncolytic mineralized bacteria as potent locally administered immunotherapeutics
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Oncolytic bacteria can trigger innate immune activity. However, the antitumour efficacy of inactivated bacteria is poor, and attenuated live bacteria pose substantial safety risks. Here we show that intratumourally injected paraformaldehyde-fixed bacteria coated with manganese dioxide potently activate innate immune activity, modulate the immunosuppressive tumour microenvironment and trigger tumour-specific immune responses and abscopal antitumour responses. A single intratumoural administration of mineralized Salmonella typhimurium suppressed the growth of multiple types of subcutaneous and orthotopic tumours in mice, rabbits and tree shrews and protected the cured animals against tumour rechallenge. We also show that mineralized bacteria can be administered via arterial embolization to treat orthotopic liver cancer in rabbits. Our findings support the further translational testing of oncolytic mineralized bacteria as potent and safe antitumour immunotherapeutics. Intratumourally administered bacteria coated with manganese dioxide modulate the immunosuppressive tumour microenvironment and potently activate antitumour immune responses, as shown in multiple solid tumours in small animals.
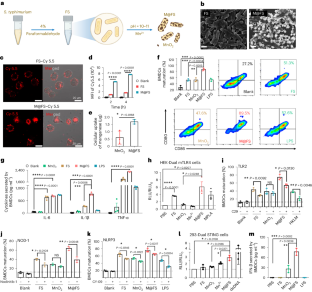

作为强效局部免疫疗法的肿瘤溶解矿化细菌。
肿瘤溶解细菌可激发先天性免疫活动。然而,灭活细菌的抗肿瘤效果不佳,而减毒活菌则存在很大的安全风险。在这里,我们展示了瘤内注射多聚甲醛固定的涂有二氧化锰的细菌能有效激活先天性免疫活性,调节免疫抑制性肿瘤微环境,并引发肿瘤特异性免疫反应和腹腔抗肿瘤反应。对小鼠、兔子和树鼩进行一次瘤内矿化鼠伤寒沙门氏菌给药,可抑制多种类型的皮下和原位肿瘤的生长,并保护治愈的动物免受肿瘤的再次侵袭。我们还发现,矿化细菌可通过动脉栓塞治疗兔子的肝癌。我们的研究结果支持将溶瘤矿化细菌进一步转化为有效、安全的抗肿瘤免疫疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


