Resilient anatomy and local plasticity of naive and stress haematopoiesis
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
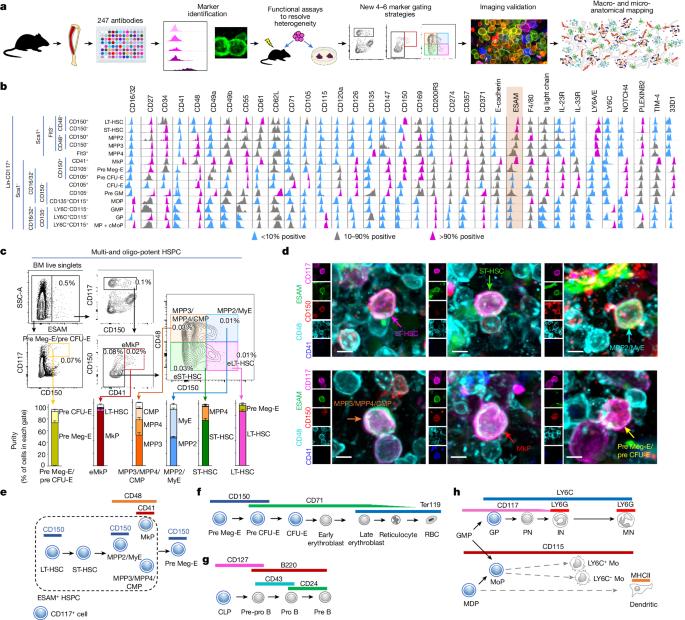
The bone marrow adjusts blood cell production to meet physiological demands in response to insults. The spatial organization of normal and stress responses are unknown owing to the lack of methods to visualize most steps of blood production. Here we develop strategies to image multipotent haematopoiesis, erythropoiesis and lymphopoiesis in mice. We combine these with imaging of myelopoiesis1 to define the anatomy of normal and stress haematopoiesis. In the steady state, across the skeleton, single stem cells and multipotent progenitors distribute through the marrow enriched near megakaryocytes. Lineage-committed progenitors are recruited to blood vessels, where they contribute to lineage-specific microanatomical structures composed of progenitors and immature cells, which function as the production sites for each major blood lineage. This overall anatomy is resilient to insults, as it was maintained after haemorrhage, systemic bacterial infection and granulocyte colony-stimulating factor (G-CSF) treatment, and during ageing. Production sites enable haematopoietic plasticity as they differentially and selectively modulate their numbers and output in response to insults. We found that stress responses are variable across the skeleton: the tibia and the sternum respond in opposite ways to G-CSF, and the skull does not increase erythropoiesis after haemorrhage. Our studies enable in situ analyses of haematopoiesis, define the anatomy of normal and stress responses, identify discrete microanatomical production sites that confer plasticity to haematopoiesis, and uncover unprecedented heterogeneity of stress responses across the skeleton. This study develops a method for spatially resolving multipotent haematopoiesis, erythropoiesis and lymphopoiesis in mice and uncovers heterogeneous haematopoietic stress responses in different bones.
天真和应激造血的弹性解剖和局部可塑性。
骨髓会调整血细胞的生成,以满足受损伤时的生理需求。由于缺乏可视化大多数造血步骤的方法,正常反应和应激反应的空间组织尚不清楚。在这里,我们开发了对小鼠多能造血、红细胞生成和淋巴生成进行成像的策略。我们将这些方法与骨髓造血1 的成像技术相结合,以确定正常和应激造血的解剖结构。在稳定状态下,单个干细胞和多潜能祖细胞分布在骨髓中,富集在巨核细胞附近。符合血系的祖细胞被招募到血管,在那里形成由祖细胞和未成熟细胞组成的特定血系微解剖结构,作为各主要血系的生成场所。这种整体解剖结构对损伤有很强的抵抗力,在大出血、全身性细菌感染、粒细胞集落刺激因子(G-CSF)治疗以及老化过程中都能保持不变。造血位点具有造血可塑性,因为它们在应对损伤时会有区别、有选择地调节其数量和输出。我们发现,骨骼的应激反应各不相同:胫骨和胸骨对 G-CSF 的反应相反,头骨在出血后不会增加红细胞生成。我们的研究实现了对造血的原位分析,确定了正常反应和应激反应的解剖结构,识别了赋予造血可塑性的离散微解剖生成位点,并发现了骨骼应激反应前所未有的异质性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


