Synthetic dual co-stimulation increases the potency of HIT and TCR-targeted cell therapies
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
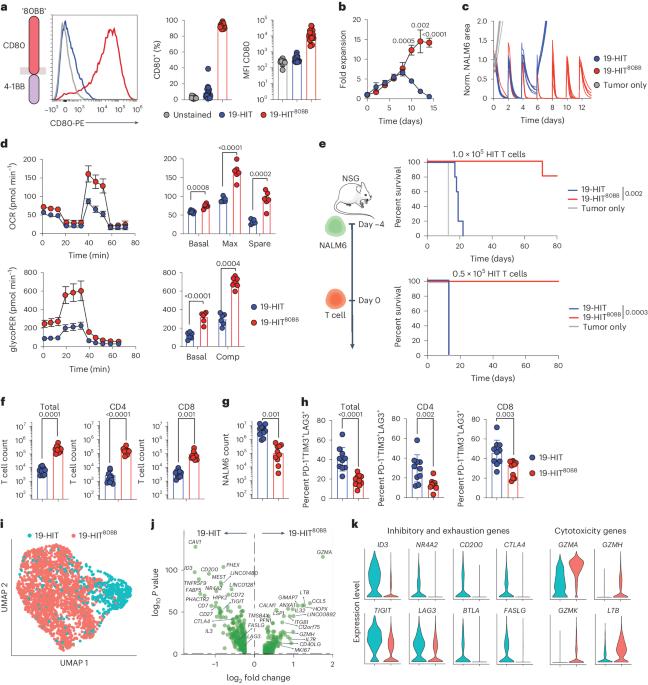
Chimeric antigen receptor T cells have dramatically improved the treatment of hematologic malignancies. T cell antigen receptor (TCR)-based cell therapies are yet to achieve comparable outcomes. Importantly, chimeric antigen receptors not only target selected antigens but also reprogram T cell functions through the co-stimulatory pathways that they engage upon antigen recognition. We show here that a fusion receptor comprising the CD80 ectodomain and the 4-1BB cytoplasmic domain, termed 80BB, acts as both a ligand and a receptor to engage the CD28 and 4-1BB pathways, thereby increasing the antitumor potency of human leukocyte antigen-independent TCR (HIT) receptor- or TCR-engineered T cells and tumor-infiltrating lymphocytes. Furthermore, 80BB serves as a switch receptor that provides agonistic 4-1BB co-stimulation upon its ligation by the inhibitory CTLA4 molecule. By combining multiple co-stimulatory features in a single antigen-agnostic synthetic receptor, 80BB is a promising tool to sustain CD3-dependent T cell responses in a wide range of targeted immunotherapies. Dobrin et al. develop a fusion receptor comprising the CD80 ectodomain and the 4-1BB cytoplasmic domain, which engages the CD28 and 4-1BB pathways and increases the antitumor potency of HLA-independent (HIT) and TCR-engineered T cells.
合成双重协同刺激可提高 HIT 和 TCR 靶向细胞疗法的效力
嵌合抗原受体 T 细胞大大改善了血液恶性肿瘤的治疗。基于T细胞抗原受体(TCR)的细胞疗法尚未取得可比的疗效。重要的是,嵌合抗原受体不仅能靶向选定的抗原,还能通过它们在识别抗原时参与的共刺激通路重编程 T 细胞功能。我们在这里展示了一种由CD80外结构域和4-1BB胞质结构域组成的融合受体(称为80BB),它既是配体又是受体,可参与CD28和4-1BB途径,从而提高人类白细胞抗原无关的TCR(HIT)受体或TCR工程T细胞和肿瘤浸润淋巴细胞的抗肿瘤效力。此外,80BB 还是一种开关受体,当它与抑制性 CTLA4 分子连接时,可提供激动性 4-1BB 协同刺激。80BB 在单一抗原识别合成受体中结合了多种协同刺激功能,是在多种靶向免疫疗法中维持 CD3 依赖性 T 细胞反应的一种很有前途的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


